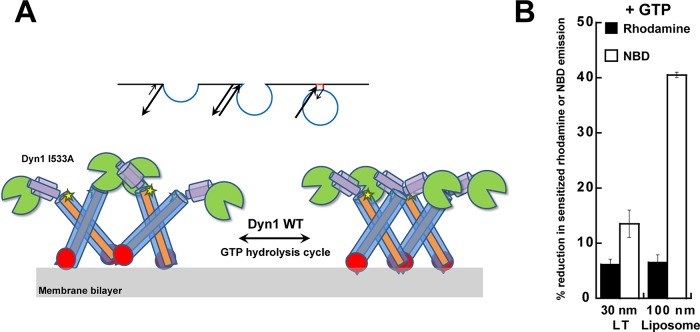
FIGURE 8:
Model for dynamin polymer dynamics and the role of GTP hydrolysis. (A) Proposed model for alternate PH-domain orientations in the regulation of dynamin polymer morphology and membrane constriction. The Dyn1 WT polymer oscillates between VL1-inserted, uniformly constricted (right) and VL1-retracted, variably relaxed (left) states during the dynamin GTP hydrolysis cycle. Dyn1 I533A is stabilized in the latter state, causing nonuniform membrane tubule constriction. The short spike in the PH-domain illustration represents VL1, and the star represents the BODIPY dye coupled to C708 in GED, which detects dynamin self-assembly in the proper register. Curvature-sensitive association of the dynamin PH domain to the progressively narrowing endocytic pit membrane neck is illustrated (inset) as a ratio of on- and off-rates (arrows). (B) Percentage reduction in BODIPY-rhodamine FRET or NBD emission intensity upon addition of GTP (1 mM) to BODIPY-Dyn1 WT or RCLDyn1 G532C-NBD (0.1 μM protein), respectively, in the presence of either LT or liposomes. BODIPY-rhodamine FRET detects alternate PH domain–membrane interactions, whereas NBD detects VL1 membrane insertion (Ramachandran et al., 2009). Note that in the constant presence of GTP, highly curved LT stabilizes VL1 membrane insertion far greater than relatively planar liposomes, underscoring the critical role of preacquired membrane curvature in the mechanism of dynamin-mediated membrane fission.