FIGURE 1.
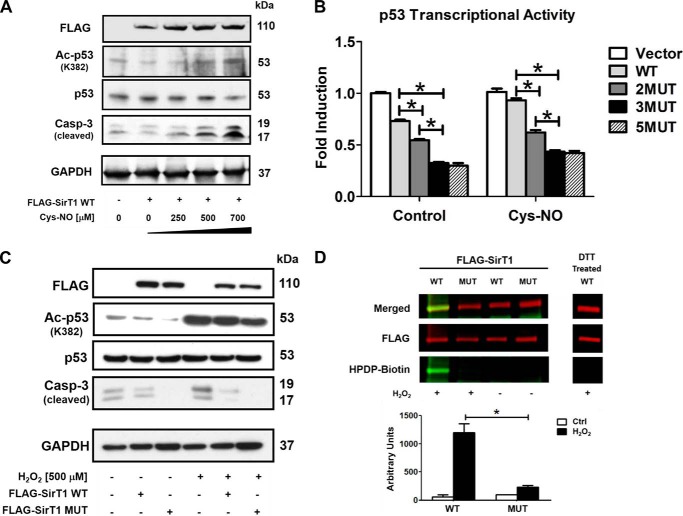
SirT1 WT inactivation by oxidative and nitrosative stress is abrogated by a triple cysteine mutant in HepG2 cells. HepG2 cells were transiently transfected for 48 h with either FLAG-SirT1 WT, FLAG-SirT1 MUT (mutated residues C61S, C318S, and C613S), or empty vector (pcDNA3.1) as a control. Equal SirT1 overexpression was confirmed by Western blot analysis with an antibody against the FLAG tag. Representative Western blots of three independent experiments are shown. A, cells were treated with Cys-NO (bolus addition) for 8 h at the indicated concentrations. Overexpression of SirT1 suppressed caspase 3 (Casp-3) cleavage and p53 acetylation (Ac-p53) at the SirT1-specific substrate site Lys382. Increased concentrations of Cys-NO inhibited the SirT1-dependent effect, increasing p53 acetylation and proapoptotic cleaved caspase 3. B, p53 transcriptional activity was measured by transfecting a PUMA-specific luciferase reporter into cells. Cys-NO (bolus addition of 500 μm) treatment inhibited the SirT1-dependent (WT) suppression of PUMA promoter activity, but the double, triple, and quintuple mutants (2MUT, 3MUT, and 5MUT) maintained PUMA suppression when treated with Cys-NO. The triple mutant (3MUT) exhibited the greatest effect and was used for the remainder of the experiments (referred to as MUT SirT1). ANOVA and Bonferroni's post-test were used (n = 3). Error bars, S.D. *, p < 0.001, WT SirT1- versus SirT1 mutant-transfected HepG2 cells either under control conditions or treated with Cys-NO. C, cells were treated with H2O2 (500 μm bolus addition) for 8 h. Overexpression of SirT1 WT reduced cleaved caspase 3 in H2O2-treated cells, whereas MUT almost restored control levels. D, reversible oxidative modifications of cysteines were detected in SirT1 WT and MUT with a biotin switch assay and IR-based (LI-COR Biosciences) Western analysis. Transfected cells were treated for 8 h with hydrogen peroxide (bolus addition) (n = 3). Cysteines of WT SirT1 were reversibly modified by H2O2 (500 μm) treatment as demonstrated by HPDP-biotin labeling of the reduced cysteine modification (green, biotin-labeled protein; red, FLAG antibody for detection of SirT1). DTT treatment preceding the labeling procedure (right panel) shows no staining, confirming the specificity of the method for reversible oxidation. For details please refer to the “Experimental Procedures.” ANOVA and Bonferroni's post-test were used (n = 3). Error bars, S.D. *, p < 0.001, WT versus MUT SirT1-transfected HepG2 cells treated with H2O2.