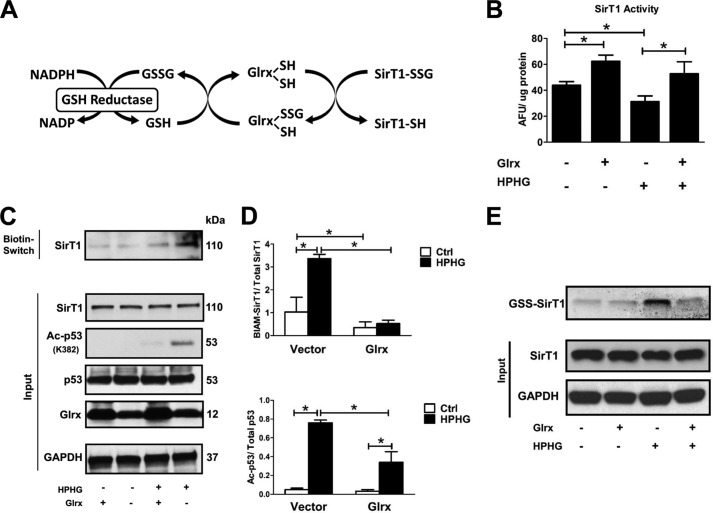
FIGURE 4.
Glrx overexpression reduces reversible oxidative modifications and maintains endogenous SirT1 activity in HPHG-treated HepG2 cells. A, protein S-glutathionylation is a dynamic reversible process that is regulated by Glrx. Glutathione-protein adducts in the cytosol and nucleus are specifically reduced by Glrx, which in a thiol exchange reaction transfers the glutathione from the target protein to itself. Glrx regenerates by reacting with free GSH to form oxidized glutathione (GSSG). Finally, oxidized glutathione is reduced to GSH by glutathione reductase, utilizing NADPH as an electron donor and reduction equivalent. B, cells were transiently transfected for 48 h with FLAG-SirT1 WT and Glrx. SirT1 was immunoprecipitated via its FLAG tag, and activity was measured by the Fluor-de-Lys assay. HPHG inhibited SirT1 activity. Overexpression of Glrx increased SirT1 activity even under control conditions and maintained its full activity in HPHG treatment. ANOVA and Bonferroni's post-test were used (n = 3). Error bars, S.D. *, p < 0.05, control versus HPHG-treated HepG2 cells; *, p < 0.01 Glrx-transfected HepG2 versus empty vector in the control or HPHG-exposed group. C, reversible oxidative cysteine modifications of endogenous SirT1 were detected by a biotin switch assay (BIAM-labeled SirT1). p53 acetylation was measured by Western blot analysis as a surrogate marker for SirT1 activity. Cells treated with HPHG for 16 h showed increased reversible oxidation of endogenous SirT1. Overexpression of Glrx by transfecting cells for 48 h decreased reversible oxidation of endogenous SirT1 and p53 acetylation. D, densitometric analysis of reversibly oxidized (BIAM-labeled SirT1) to total SirT1 and acetylated p53 (Ac-p53) to total p53 in Glrx-overexpressing cells. ANOVA and Bonferroni's post-test were used (n = 3). Error bars, S.D. *, p < 0.001, control versus HPHG-treated HepG2 cells and empty vector versus Glrx-transfected in control or HPHG-exposed HepG2 cells. E, HepG2 cells were incubated with the cell-permeable and biotin-labeled glutathione monoethyl ester (40 μm). GSH adducts on SirT1 were detected by Western blot with streptavidin. HPHG exposure of cells increased SirT1-GSH adducts, which were prevented or reversed by overexpression of Glrx. Ctrl, control; AFU, arbitrary fluorescent units.