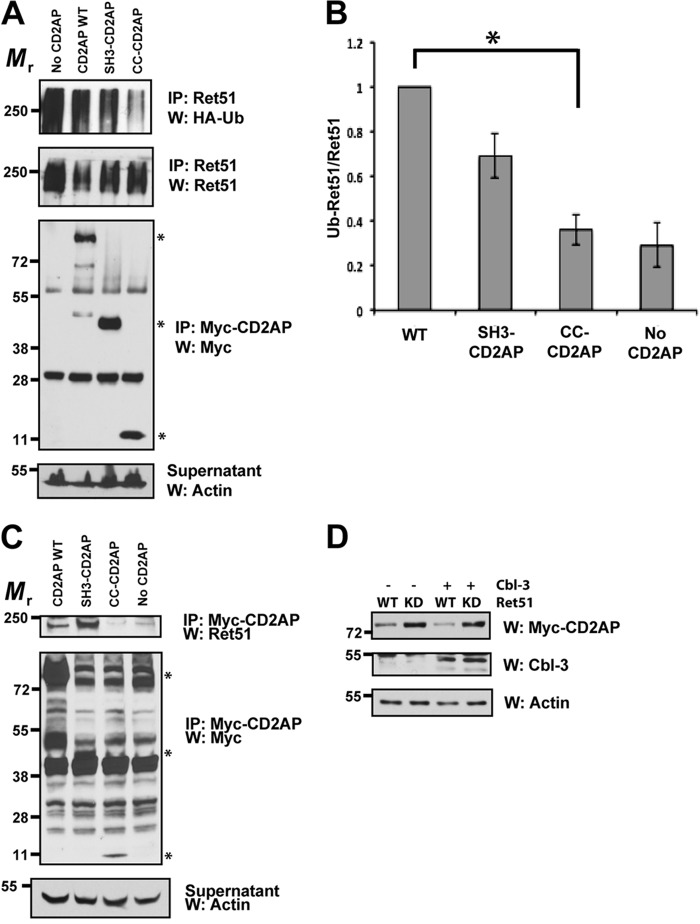
FIGURE 6.
The SH3 domains of CD2AP are sufficient for Ret association and Cbl-3-mediated ubiquitination. A, cells were transfected with full-length CD2AP, with the amino-terminal three SH3 domains of CD2AP (1–328 amino acids, SH3-CD2AP) or the carboxyl-terminal 120 amino acids that encodes the coiled-coil domain of CD2AP (561–681, CC-CD2AP). All of the cells were also cotransfected with Cbl-3, Ret51, and HA-ubiquitin. Ret ubiquitination was ascertained as in Fig. 1E. Supernatants from the first Ret immunoprecipitation were subsequently immunoprecipitated using anti-Myc and probed for Myc to confirm similar expression of the CD2AP variants. Asterisks on the right side of the Myc-CD2AP blot indicate the position of full-length CD2AP and the two truncations of CD2AP. B, the experiments in A were quantified and graphed as the means ± S.E. (n = 4). The asterisk indicates a significant difference between the WT CD2AP and CC-CD2AP conditions, p < 0.05. C, NIH3T3 cells were transfected with the same plasmids as in A. The cells were then subjected to Myc (CD2AP) immunoprecipitation followed by immunoblotting for Ret51. Myc immunoblotting of the immunoprecipitation supernatants confirmed the expression of the CD2AP variants (middle panels). The relative molecular masses of CD2AP, SH3-CD2AP, and CC-CD2AP are indicated with asterisks as in A (79–90, 45–55, and 10–15 kDa, respectively). Actin served as a loading control. D, NIH3T3 cells were transfected with Ret51 (WT) or kinase inactive Ret51 (KD) along with CD2AP, with or without Cbl-3. After 24 h, detergent extracts were produced from the transfected cells, and these extracts were immunoblotted with antibodies to CD2AP (Myc), Cbl-3, or actin as a loading control. The cells were not treated with protease inhibitors such that differences in protein degradation could be observed. The experiments in this figure were performed two or three times with similar results. IP, immunoprecipitation; W, Western blot.