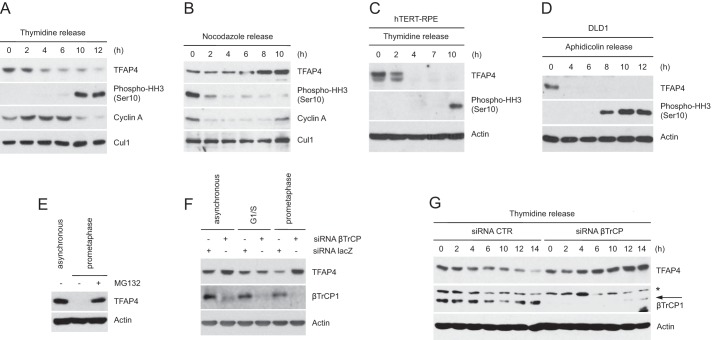
FIGURE 3.
TFAP4 is degraded during the G2 phase of the cell cycle. A and B, HeLa cells were synchronized at the G1/S transition by double thymidine block and then released in fresh medium containing nocodazole (A) or in mitosis by the nocodazole block-and-release procedure (B). C, hTERT-RPE1 cells were synchronized at G1/S by double thymidine block and released into nocodazole containing medium. D, to synchronize DLD1 cells at G1/S, cells were first serum starved (32 h) and then incubated in serum-containing medium in the presence of aphidicolin for 24 h. Cells were then released from the G1/S block in nocodazole-containing medium. Cells were collected at the indicated times, lysed, and analyzed by immunoblotting with the indicated antibodies. Actin or Cul1 were used as loading controls. E, asynchronously growing or nocodazole-treated HeLa cells were collected and analyzed by immunoblotting with antibodies for the indicated proteins. When indicated, cells were treated with the proteasome inhibitor MG132 for 6 h. F, HeLa cells were transfected with siRNA corresponding to a non-relevant mRNA (control siRNA) or to βTrCP mRNA. Twelve hours after transfection, cells were either left untreated of treated with thymidine (to synchronize them at G1/S) or nocodazole (to synchronize them in prometaphase). Cells were collected, lysed, and analyzed by immunoblotting. G, HeLa cells, transfected as in F, were synchronized as in A. Cells were then collected at the indicated time points and analyzed by immunoblotting with antibodies for the indicated proteins. Actin levels are shown as loading control. The asterisk indicates a nonspecific band.