FIGURE 2.
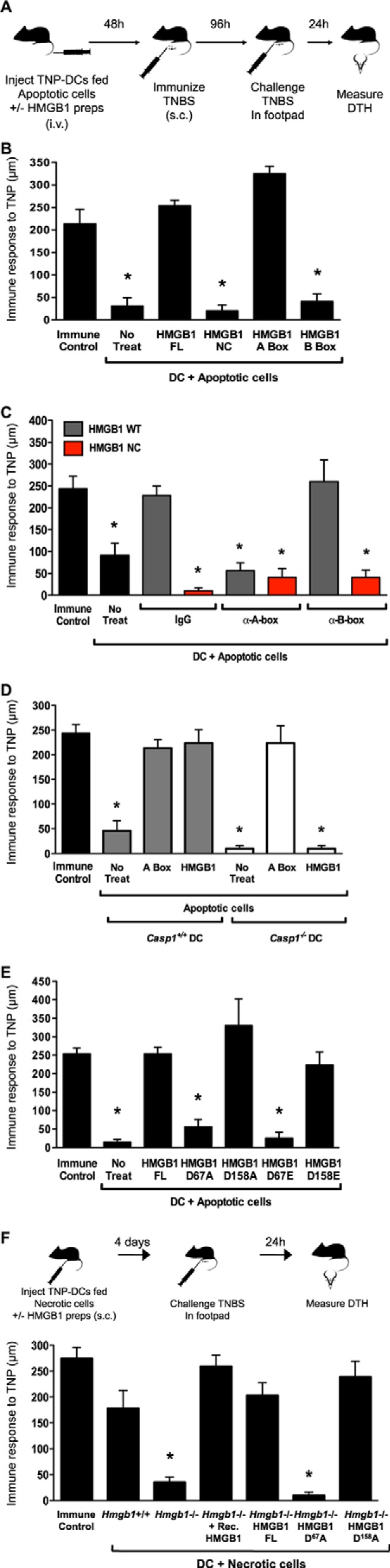
HMGB1 reverses apoptosis-induced tolerance via the A-box. A, schematic representation of the DTH assay. TNP-conjugated DCs fed apoptotic cells in the absence or presence of exogenous recombinant HMGB1 preparations were injected intravenously (i.v.). Mice were immunized 2 days later by subcutaneous (s.c.) injection of TNBS. Four days following immunization, mice were injected with TNBS in the right footpad and PBS in the left footpad. DTH was measured with a micrometer 24 h later as the difference in thickness between the right and left footpads. Immune control mice were injected with TNBS subcutaneously and challenged in the footpads. B, full-length WT (FL) HMGB1 and the A-box but not full-length NC HMGB1 or the B-box reverse tolerance in DTH. Equimolar amounts of recombinant HMGB1 proteins were incubated with TNP-conjugated WT CD8α+ DCs fed apoptotic splenocytes. The tolerogenic activity of the treated DCs was examined in the DTH assay. No treat, no treatment. C, neutralization of HMGB1 A-box reverses the effects of HMGB1 effects in blocking tolerance. DTH tolerance assay was conducted as in B with culture media supplemented with IgG control or neutralizing antibodies targeting the A-box or B-box. D, DC expression of caspase-1 is required for HMGB1 activation and reversal of tolerance in DTH. Equimolar amounts of recombinant full-length HMGB1 or the A-box were incubated with TNP-conjugated WT or Casp1−/− CD8α+ DCs fed apoptotic splenocytes. The tolerogenic activity of the treated DCs was examined in the DTH assay. E, as in B using full-length WT HMGB1 and HMGB1 mutated at Asp67 or Asp158 to Ala or Glu. F, top, schematic representation of the DTH priming assay. TNP-conjugated DCs fed necrotic cells in the absence or presence of exogenous recombinant HMGB1 preparations were injected subcutaneously in mice. Four days later, mice were injected with 10 mm TNBS in the right footpad and PBS in the left footpad. DTH was measured with a micrometer 24 h later. Immune control mice were injected with 10 mm TNBS subcutaneously. Bottom, Hmgb1+/+ MEFs, Hmgb1−/− MEFs, or Hmgb1−/− MEFs stably transfected with full-length WT HMGB1 (FL), D67A, or D158A mutants were exposed to 10 mm TNBS, made necrotic, and fed to WT CD8α+ DCs overnight in the absence or presence of recombinant HMGB1. DTH responses were measured as the DCs ability to prime immunity to TNBS.
