FIGURE 5.
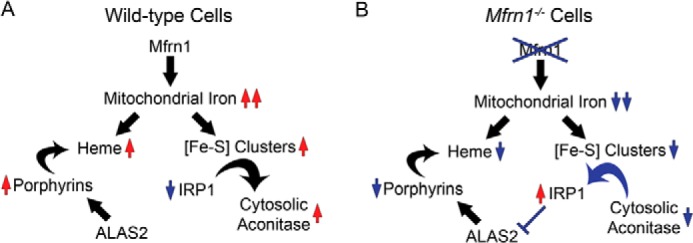
Schematic model of mitochondrial heme and Fe/S cluster co-regulation. A, MFRN1 imports iron into the mitochondria for heme and [Fe-S] cluster biosynthesis. When mitochondrial iron is readily available, sufficient [Fe-S] cluster synthesis converts IRP1 from an IRE-binding protein to cytosolic aconitase. B, however, when mitochondrial iron stores are compromised by MFRN1 mutation, both heme and [Fe-S] cluster syntheses are disrupted. Deficient [Fe-S] cluster availability results in activation of IRP1 that subsequently binds to IREs on target transcripts. IRP1 binding to the 5′-IRE of Alas2 mRNA inhibits ALAS2 translation, thereby forming a feedback loop that attenuates protoporphyrin accumulation in the absence of mitochondrial iron.
