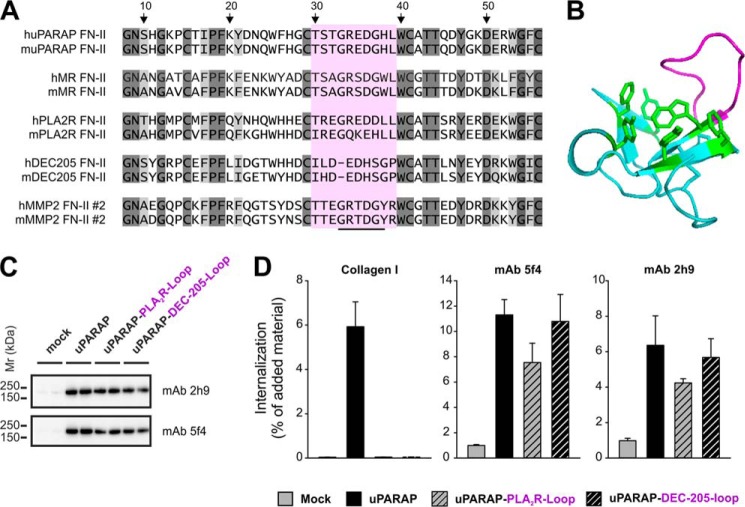
FIGURE 7.
Loss of collagen internalizing function in uPARAP FN-II mutants. A, list of sequences representing the second FN-II domain from human and mouse MMP-2 and the single FN-II domain from human and mouse uPARAP, MR, PLA2R, and DEC-205. Dark gray: amino acids completely retained in all sequences. Light gray: amino acid positions where only conservative changes have occurred. The stretch of amino acids (Gly33–Tyr38) suggested to be involved in collagen binding is underlined in the MMP-2 sequence. The sequence marked by purple (Thr30–Arg39) constitutes a loop protruding from the core of the second FN-II domain from MMP-2. The amino acid numbering refers to the position in the second FN-II domain sequence from MMP-2 following published FN-II domain annotation (38). B, crystal structure of the ligand free form of the second FN-II domain from MMP-2 (PDB ID: 1CK7)(53). The protruding loop including Gly33–Tyr38 is marked by purple. The aromatic side chains of residues forming a hydrophobic groove, also suggested to be important in ligand binding, are marked by green. C, Western blot analysis of uPARAP loop mutants (uPARAP-PLA2R-Loop and uPARAP-DEC-205-Loop) expression in HEK-293T cells. Anti-uPARAP mAb 2h9 or 5f4 were used as primary antibodies for detection. Experimental conditions were as described in Fig. 2. D, internalization of radiolabeled collagen type I (100 ng/ml, left panel) or control ligands (100 ng/ml mAb 5f4 or 2h9, center and right panel, respectively) by HEK-293T cells transfected with uPARAP loop mutants. Experimental conditions and data presentation were as described for Fig. 6.