FIGURE 2.
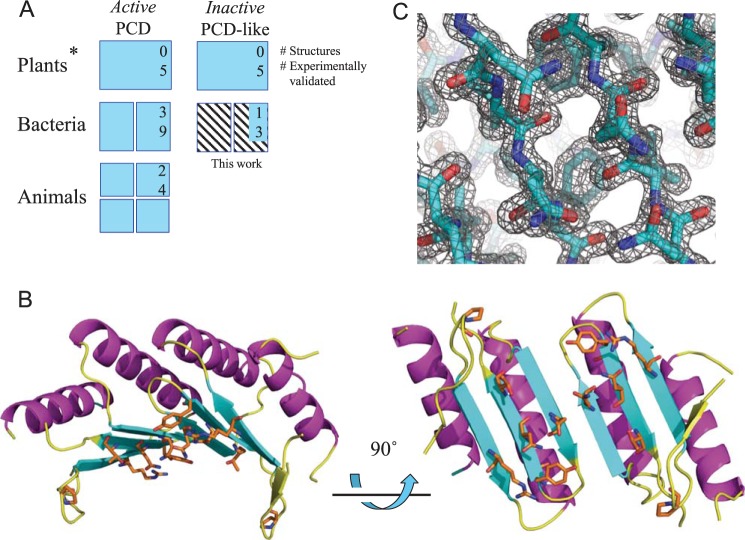
Crystal structure and conserved surface regions in the acRAF dimer. A, diagram summarizing current knowledge of PCDs and inactive PCD-like proteins. PCDs are capable of pterin-4α-carbinolamine dehydratase activity. PCD-like proteins, whereas structurally similar to PCDs, do not show evidence of pterin-4α-carbinolamine dehydratase activity. Oligomeric states are represented by number of subunits shown, except for plant PCD and PCD-like proteins (denoted by *), whose oligomeric states are unknown. Numbers in upper right hand corners represent the number of representative proteins with crystal structures deposited in the PDB. Numbers below indicate the number of representative proteins examined experimentally, to our knowledge. Information on the bacterial carboxysomal acRAFs (hatched) refers to the present study. B, solvent-exposed, conserved residues of the acRAF dimer are shown as orange sticks. C, σA-weighted 2mFo − DFc electron density map of the dimer interface viewed in the same orientation as the bottom right model in panel B.