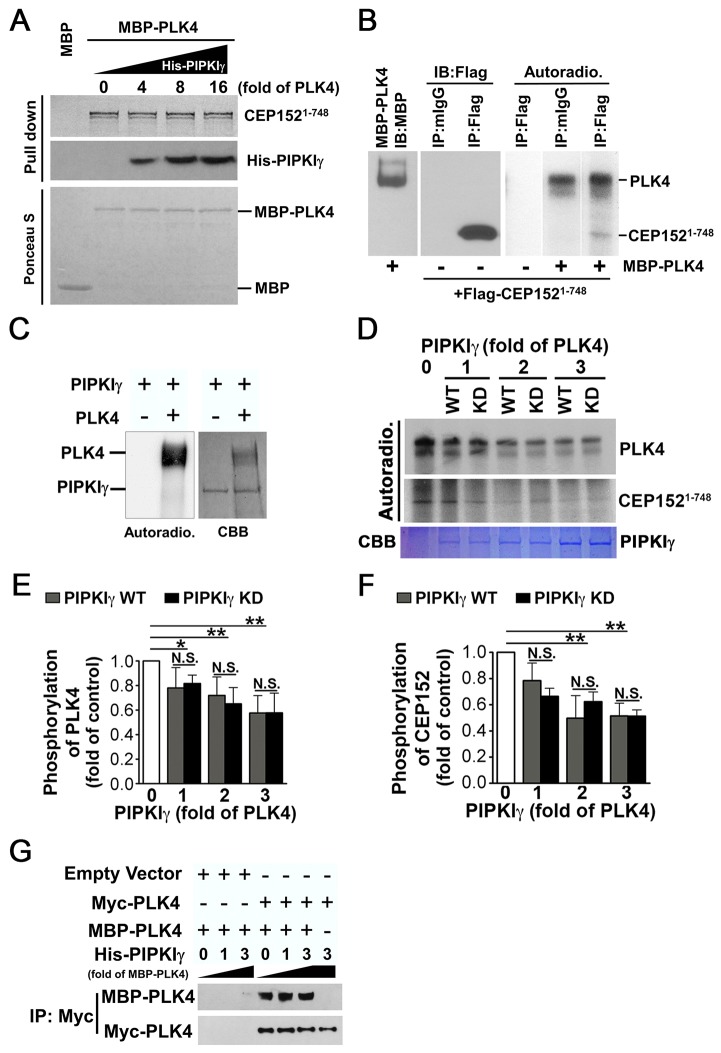
Fig. 7.
The binding of PIPKIγ impairs PLK4 kinase activity. (A) PIPKIγ does not affect the interaction between CEP152 and PLK4. The [35S]Met-labeled N-terminus of CEP152 (CEP1521-748) was expressed using an in vitro transcription/translation system. Its interaction with MBP–PLK4 was determined with or without the presence of the indicated amount of His–PIPKIγ, using a MBP-pull-down assay. The loading of MBP and MBP–PLK4 is shown by Ponceau S staining. (B) PLK4 phosphorylates both itself and CEP152. A FLAG-tagged CEP152 fragment (CEP1521-748) was overexpressed in HeLa cells and immunoprecipitated with anti-FLAG antibody. It was then used in a PLK4 kinase assay as a substrate for purified MBP–PLK4. Lane 1, immunoblotting (IB) showing the loading of purified MBP–PLK4. Lanes 2 and 3, IB with anti-FLAG antibody to analyze the immunoprecipitates pulled down by mouse IgG (mIgG) or anti-FLAG antibody. Lanes 4–6, autoradiographs (Autoradio.) showing the phosphorylation of PLK4 and CEP1521-748 when CEP1521-748 was incubated with or without MBP–PLK4 in the PLK4 kinase assay. (C) PLK4 could not phosphorylate PIPKIγ. The left panel shows autoradiographs of the PLK4 kinase assay when purified His–PIPKIγ was incubated with or without MBP–PLK4. The right panel shows Coomassie Brilliant Blue (CBB) staining indicating the loading of MBP–PLK4 and His–PIPKIγ. (D) PIPKIγ inhibits PLK4 activity. PLK4 autophosphorylation or PLK4-meditated phosphorylation of CEP1521-748 was determined using the PLK4 kinase assay with or without purified wild-type (WT) or kinase-dead (KD) PIPKIγ as indicated. The upper panel shows the results of autoradiography of phosphorylated PLK4 or CEP1521-748. The lower panel shows the loading of PIPKIγ proteins. (E,F) Quantification of phosphorylated PLK4 (E) or CEP1521-748 (F) from three independent experiments. A single asterisk (*) represents P<0.05; a double asterisk (**) represents P<0.01; N.S., no significant difference. (G) PIPKIγ does not affect the dimerization of PLK4. HeLa cells expressing either control construct or Myc–PLK4 construct were subjected to immunoprecipitation using an anti-FLAG antibody. A pull-down assay was used to determine the co-precipitation of the resulting immunoprecipitates with purified soluble MBP–PLK4 with or without an increasing amount of His−PIPKIγ. The final precipitates were then blotted with the indicated antibodies.