Fig. 1.
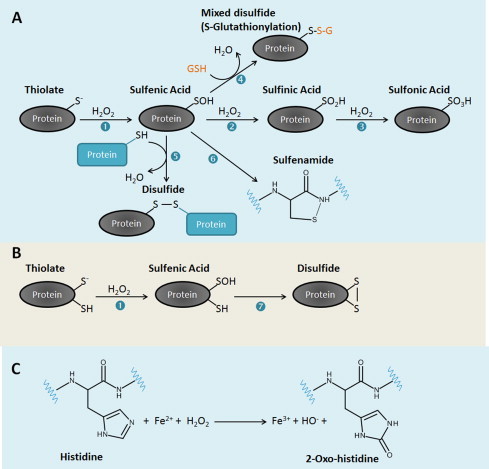
Oxidative modifications of cysteine (A and B) and histidine (C) residues in proteins induced by H2O2. In cells, sulfhydryl (SH) groups of cysteine residues with low pKa may ionize forming thiolates. Thiolates are good nucleophiles and form a sulfenic acid (SOH) upon reaction with H2O2 (reaction 1). Once formed, the SOH can be reduced to a disulfide by a reaction with the SH group of another cysteine residue either in the same (reaction 7) or in a second protein (reaction 5). Alternatively, a SOH can react with the low molecular weight thiol glutathione (GSH) (reaction 4) to form a mixed disulfide in a reaction known as S-glutathionylation or S-thiolation. In an event where a neighboring cysteine residue or GSH is absent, the amide nitrogen of a neighboring amino acid residue can attack the SOH to form a sulfenamide (reaction 6). This reaction occurs in PTP1B. The SOH can also react further with H2O2 to generate more oxidized forms of sulfur, the sulfinic acid (SO2H) (reaction 2) and sulfonic acid SO3H (reaction 3). Disulfides can be reduced back to thiols using the thioredoxin/thioredoxin reductase and glutaredoxin/GSH/glutathione reductase systems. Sulfinic acids in 2-cys Prxs, but not other proteins, can be reduced to thiols using the enzyme sulfiredoxin [372]. No known enzyme is able to catalyze the reduction of sulfonic acids in proteins. In proteins containing iron metal centers such as PerR, histidine residues can be oxidized by H2O2 in a Fenton-like reaction possibly involving the formation of the hydroxyl radical as an intermediate, to form 2-oxo-histidine.
