Fig. 7.
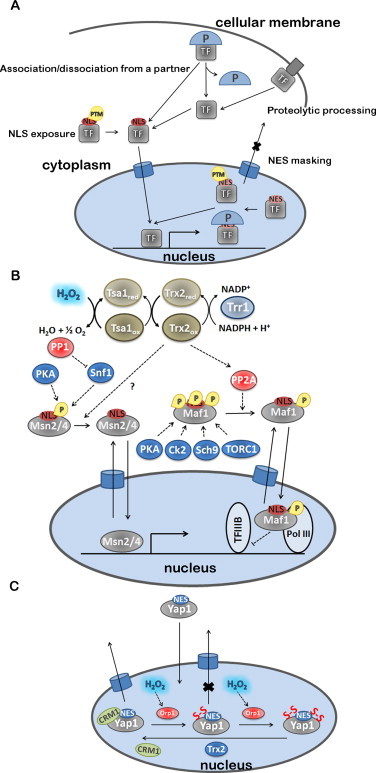
Regulation of cytoplasm-nuclear trafficking of TF by H2O2. (A) Nuclear localization of TF is essential for gene expression activation and H2O2 plays a key role in TF cellular trafficking. H2O2 modulates NLS exposure by removing PTM or by promoting partner dissociation (P). In certain cases, the association to adaptor proteins might promote NLS exposure. Inversely, NES masking is another mechanism to retain TF in the nucleus that is mediated by H2O2. Conformational changes induced by PTM together with the formation of protein complexes make NES inaccessible inducing activation of transcription. Other TF are associated to cellular membranes in their inactive state. The activation of these TF requires proteolytic cleavage and release to the cytoplasm where it is transported to the nucleus. (B) Msn2/4 and Maf1 translocation to the nucleus is activated after dephosphorylation, which uncovers NLS. This process is activated by H2O2 indirectly through Trx system. Msn2/4 and Maf1 dephosphorylation is dependent upon Trx2 by an unknown mechanism and by PP2A activation, respectively. (C) Yap1-dependent gene activation depends on its retention in the nucleus by CRM1 dissociation that occurs in presence of H2O2. The oxidation of four Cys residues in Yap1 is responsible for the conformational alterations that prevent NES recognition by CRM1. As for Msn2/4 and Maf1, Yap1 does not react directly with H2O2, and its oxidation is mediated by a GPx, Orp1. Trx2 reduces Yap1 inducing its translocation to the cytoplasm, inactivating gene transcription. Factors colored blue are inhibitors of TF-dependent gene expression; factors colored red are activators of TF-dependent gene expression. Dashed lines indicate activation/inhibition.
