Figure 2.
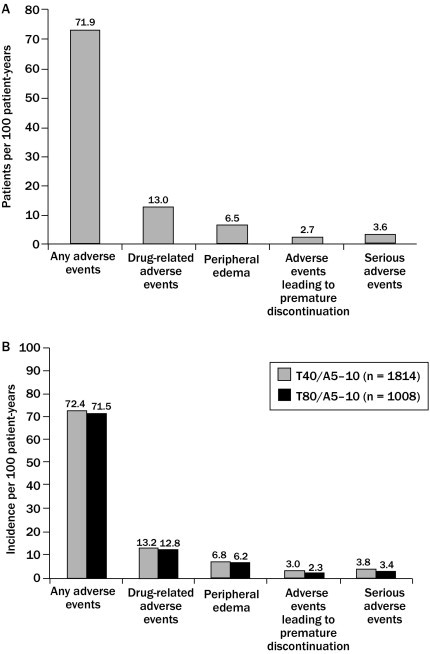
Comparison of the duration-adjusted (A) patients per 100 patient years and (B) incidence per 100 patient years of drug-related adverse events, peripheral edema, adverse events leading to premature discontinuation, and serious adverse events in patients receiving long-term, open-label treatment with telmisartan 40 mg/amlodipine 5–10 mg (T40/A5–10) and patients receiving telmisartan 80 mg/amlodipine 5–10 mg (T80/A5–10) (data for TEAMSTA-5 [n = 1814] and −10 [n = 1008] Follow-Up; pooled post-hoc analysis).
