Abstract
The ability of cells to generate a highly polarized intracellular signal through G protein-coupled receptors (GPCRs) is essential for their migration toward chemoattractants. The Gβγ subunits of heterotrimeric G proteins play a critical role in transmitting chemotactic signals from GPCRs via the activation of diverse effectors, including PLCβ and PI3K, primarily at the leading edge of cells. Although Gβγ can directly activate many of these effectors through protein-protein interactions in vitro, it remains unclear how Gβγ spatially and temporally orchestrates the activation of these effectors in vivo. A yeast two-hybrid screen for Gβ interacting proteins identified two WD40-repeat domain containing proteins, RACK1 and WDR26, which are predicted to serve as scaffolding/adaptor proteins. Previous data indicates that RACK1 negatively regulates Gβγ-mediated leukocyte migration by inhibiting Gβγ-stimulated PLCβ and PI3K activities. In contrast, recently published work by Sun et al. indicates that WDR26 promotes leukocyte migration by enhancing Gβγ-mediated signal transduction. These findings reveal a novel mechanism regulating Gβγ signaling during chemotaxis, namely through the positive and negative regulation of WDR26 and RACK1 on Gβγ to promote and fine tune Gβγ-mediated effector activation, ultimately governing the ability of cells to polarize and migrate toward a chemoattractant gradient.
Keywords: chemotaxis, G proteins, WD40 proteins, signal transduction, G protein-coupled receptors
Directed cell migration along chemoattractant gradients, termed chemotaxis, plays a crucial role in normal physiological and pathological processes. Chemotaxis is required for embryonic development, wound healing, immune surveillance and immune response.1 However, excessive or dysregulated chemotaxis promotes disease, including inflammatory disorders such as asthma and rheumatoid arthritis, formation of arterial plaques during atherosclerosis and cancer metastasis.2 Thus, understanding chemotaxis has important ramifications for both physiology and pathology, and has been the subject of intense study.
Motile cells have an amazing ability to navigate effectively in the direction of a very shallow chemoattractant gradient, with as small as 1–3% concentration difference between either end of the cell. This has been attributed to their ability to translate the shallow extracellular gradient into a much steeper intracellular gradient, which acts as an internal “compass” to steer cell migration.3 Recent studies from model systems such as Dictyostelium amoeba and neutrophils have begun to uncover the underlying molecular mechanisms responsible for this development of an intracellular “compass.” These include spatial and temporal activation of diverse signaling pathways and numerous positive and negative feedback loops acting at the front and back of cells.4,5 Nevertheless, despite these advances, many components of the signaling pathways activated by chemoattractants remain to be defined, and the mechanistic details of many feedback regulatory pathways on the chemotactic signal remain to be elucidated.
Chemoattractants act on cell surface receptors to regulate chemotaxis. Among these are G protein coupled receptors (GPCRs) that transmit chemotactic signals through heterotrimeric G proteins. In leukocytes, chemotaxis is initiated by the binding of a diverse family of small proteins, called chemokines, or the bacterial by-product fMLP to their cognate receptors and the activation of two classes of G proteins, Gi/o and G12/13.5 Activated Gi/o proteins release free Gβγ to activate diverse effectors that transmit chemotactic signals, ultimately facilitating actin polymerization at the leading edge to drive forward movement.5 In contrast, activation of G12/13 mediates actomyosin complex formation via a Rho guanine exchange factor (RhoGEF) and RhoA dependent pathway at the posterior of cells to facilitate tail retraction.6 The Gβγ-mediated signaling mechanisms controlling leading edge formation are highly conserved in many types of motile cells, including Dictyostelium amoeba and leukocytes,1,5 although the mechanisms underlying actomyosin formation differ among different cell types.7
Following Gβγ activation, an intracellular signal gradient is established that likely acts as an internal “compass” for directing cell polarization and migration. Gβγ-mediated phosphatidylinositol 3-kinase (PI3K) activation was the first pathway found to play a critical role in generating this intracellular signal.8,9 PI3K activation releases phosphotidyl inositol (3,4,5) phosphate (PIP3), which accumulates asymmetrically at the leading edge of chemotaxing cells.5 This in turn recruits RhoGEFs such as DOCK2 and αPix, which activate Rac and Cdc42 to promote F-actin polymerization and cell polarization.10,11 In recent years, much work has gone into identifying the mechanisms underlying spatially localized activation of PI3Ks. In Dictyostelium and neutrophils, amplification of the PIP3 signal is mediated by the reciprocal translocation of PI3K to the leading edge and phosphatases, such as phosphatase and tension homology (PTEN), to the lateral and trailing edge of cells.8,12,13 Moreover, the subsequent activation of Rac by PI3K creates a positive feedback loop which further enhances PI3K activation at the leading edge via F-actin polymerization.14 Additionally, G12/13-mediated RhoA activation antagonizes Gβγ-mediated PI3Kγ activation at the posterior of neutrophils, reinforcing PI3K activity at the leading edge.6 In addition to the PI3K pathway, several other pathways, including those mediated by PLCβ2/3, PLA2, p38 MAPK, pREX1 and mTORC2 have now been shown to act in parallel or in concert with the PI3K pathway to regulate leukocyte migration.15-20 These pathways are all activated downstream of Gβγ. However, beside PI3Kγ, PLCβ2/3 and pREX1, which are directly activated by Gβγ, the biochemical nature of the other pathways involved in leukocyte chemotaxis remains poorly defined because the direct binding targets of Gβγ have not been identified.
It is now well established that Gβγ functions as a master switch to transmit chemotactic signals during the migration of various cell types, including Dictyostelium and leukocytes.1,5 Nonetheless, it remains unclear how Gβγ orchestrates the activation of diverse effectors to generate the spatiotemporal intracellular signal that directs cell polarization and migration. Studies in vitro have shown that Gβγ can directly activate a number of downstream effectors, including PI3Kγ and PLCβ2/3, by direct protein-protein interactions, but how Gβγ actually regulates these effectors in vivo remains to be resolved.21 For example, both PI3Kγ and PLCβ2/3 are known to reside in the cytosol of resting cells and are rapidly translocated to the leading edge of chemotaxing cells upon stimulation with chemoattractants.18,22 The mechanism regulating the asymmetric accumulation of these proteins at the leading edge remain unclear, but is unlikely due to anchoring through Gβγ because Gβγ and chemoattractant receptors remain largely uniformly distributed in the cell membrane.9,23 Thus, there may be other regulatory mechanisms acting upon Gβγ and/or downstream effectors of Gβγ to control PI3Kγ and PLCβ2 activation.
In an attempt to identify novel Gβγ interacting proteins, a yeast two-hybrid screen was performed using Gβ as bait. Interestingly, this screen identified a number of WD40-repeat containing proteins as interacting with Gβγ, including RACK1 (receptor for activated C kinase 1) and WDR26.24 This finding was intriguing, as Gβγ is considered to be the prototypical WD40 protein, and has never before been shown to interact with other WD40 proteins.
WD40-repeat containing proteins are a highly conserved family of proteins, characterized by the presence of a 40 to 60 repeated amino acid sequence containing Trp-Asp dipeptides, which form a circular β propeller.25 The WD40 repeat domain does not appear to have any intrinsic enzymatic activity. Rather, the multi-sided propellers may serve as a scaffold to mediate protein-protein interactions.25 Indeed, Gβ is considered to be a scaffold as it has a long list of interacting proteins involved in diverse signaling networks.21 Although RACK1 was initially identified as a receptor for activated protein kinase C, it was later shown to be an adaptor/scaffold for a variety of proteins, including enzymes such as Src, FAK and PDE4D5, and membrane proteins such as receptors for NMDA, IP3, insulin-like growth factor 1, thromboxane and type 1 interferon.26 Not surprisingly, RACK1 was found to be involved in diverse physiological and pathological functions including development, cell migration, circadian rhythm and cancer.26 Previous studies suggest WDR26 may also function as a scaffold/adaptor as it was identified as one of many WD40 proteins associated with the cullin 4(CUL4)-DNA damage-binding protein 1 (DDB1) ubiquitin E3 ligase complex.27 However, the exact function of WDR26 remains elusive. Gβγ is known to recruit scaffold proteins such as Ste5p to promote the MAPK signaling cascade for pheromone response in yeast.28 The findings that RACK1 and WDR26 interact with Gβγ raise the possibility that they may serve as scaffold/adaptor proteins for orchestrating the signaling specificity and efficacy of Gβγ.
Initial studies have characterized the Gβγ-RACK1 interaction and have shown that RACK1 acts as a negative regulator in Gβγ-mediated signal transduction and leukocyte migration.29-31 Downregulation of RACK1 by siRNAs enhanced SDF1α- and fMLP-stimulated chemotaxis of Jurkat T cells and differentiated HL60 cells, whereas overexpression of RACK1 inhibited cell migration.31 The effect of RACK1 on chemotaxis may be due to its regulation of Gβγ signaling, because SDF1α- and fMLP-stimulated PLCβ and PI3K activities were significantly augmented by RACK1 downregulation, whereas RACK1 overexpression had the opposite effect. Importantly, overexpression of a fragment of RACK1 that retained the Gβγ-binding mimicked the inhibitory effect of the full-length RACK1 on Gβγ signaling and leukocyte chemotaxis.31 In vitro studies with purified proteins showed that RACK1 can directly inhibit Gβγ-mediated PLCβ and PI3Kγ activation.30,31 This inhibition was probably a result of competitive binding of RACK1 with PLCβ and PI3Kγ for a common binding site located on the side-surface of Gβγ.30,31 Intriguingly, following stimulation by chemoattractants, RACK1 was found to translocate from the cytosol to the membrane and accumulate at the leading edge of leukocytes.29,31 Given that activation of PLCβ and PI3Kγ by Gβγ at the leading edge is part of the “compass” mechanisms that generate the internal gradient of signaling molecules required for leukocyte polarization and chemotaxis, the exact role of RACK1 in the formation of the signal gradient at the leading edge is not immediately clear. Nevertheless, the fact that a negative regulator is recruited to the leading edge by Gβγ raises the intriguing possibility that a positive regulator may be also recruited by Gβγ to augment its signal during chemotaxis.
The data presented in our recently published paper by Sun et al. suggests that WDR26 may fulfill the role of a positive regulator of Gβγ signaling during leukocyte migration.32 Similar to RACK1, WDR26 was found to be rapidly translocated to the leading edge of leukocytes upon stimulation with chemoattractants. However, unlike RACK1, knockdown of WDR26 resulted in decreased leukocyte migration in vitro and homing to lymphoid tissues in vivo. By monitoring the migratory behaviors of dHL60 cells deficient in WDR26, it was found that suppression of WDR26 led to reduced cell migration speed and loss of directionality. In contrast to RACK1 inhibition, downregulation of WDR26 by siRNAs alleviated PLCβ-mediated calcium mobilization and PI3K-stimulated AKT phosphorylation in both Jurkat T cells and dHL60 cells. The effect of WDR26 was likely mediated by its binding to Gβγ because overexpression of a WDR26 fragment that mediates Gβγ binding recapitulated the effect of WDR26 knockdown on Gβγ signaling and cell migration. Together, these data indicate that WDR26 positively regulates Gβγ signaling during leukocyte migration.
The molecular mechanisms by which WDR26 facilitates Gβγ signaling are not yet clear. Our unpublished data indicate that in addition to binding Gβγ, WDR26 also directly interacts with select Gβγ effectors such as PLCβ2. Moreover, WDR26 is able to assemble a signaling complex containing both Gβγ and PLCβ2 and promote PLCβ2 activation by Gβγ, both in vitro and in vivo. Additionally, WDR26 is required for membrane translocation and accumulation of PLCβ2 at the leading edge of polarized neutrophils induced by chemoattractant stimulation. Together, these findings indicate that WDR26 functions as a scaffolding protein to promote membrane translocation and activation of Gβγ effectors.
Our data provides a number of new insights into both our understanding of leukocyte migration and the regulation of signaling downstream of Gβγ. The novel regulation of Gβγ by WDR26 and RACK1 may explain why, despite global activation of GPCRs and the heterotrimeric G protein at the periphery of the cell, activation of Gβγ effectors selectively occurs at the leading edge.1 The rapid translocation of WDR26 to the leading edge after cell stimulation likely facilitates translocation and activation of select Gβγ effectors to promote leading edge formation (Fig. 1). The recruitment of RACK1 may act in a feedback loop to restrict or fine tune the activation of such effectors, to efficiently allow for gradient formation and subsequent directed migration. Consistent with this hypothesis, co-immunoprecipitation studies showed that upon chemoattractant stimulation, WDR26 rapidly forms a complex with Gβγ (within five minutes of stimulation), whereas the interaction of RACK1 with Gβγ was delayed, occurring nearly thirty minutes after stimulation.31,32 Moreover, downregulation of WDR26 blocked the interaction of RACK1 with Gβγ and its ability to negatively regulate Gβγ-mediated cell migration, indicating that RACK1 functions downstream of WDR26.32
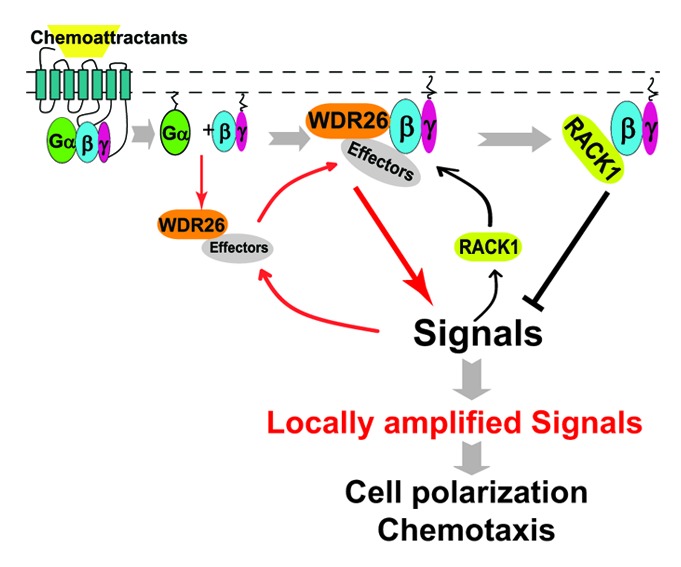
Figure 1. Working model for WDR26 and RACK1 mediated positive and negative regulation of Gβγ signaling at the cell’s leading edge during chemotaxis. In untreated cells, WDR26 binds select Gβγ effectors in the cytosol. Upon chemoattractant-mediated stimulation of Gi-coupled GPCRs, WDR26 and effectors are recruited to the membrane by Gαi/o and/or Gβγ-mediated signaling. Upon membrane recruitment, free Gβγ and WDR26 form a complex to promote Gβγ-mediated signal transduction, which may in turn lead to the recruitment of RACK1 from the cytosol. RACK1-binding to Gβγ inhibits Gβγ-mediated signal transduction by competing off either the Gβγ effectors, or the effectors in complex with WDR26. The combined activities of WDR26 and RACK1 serve to promote and fine tune Gβγ signaling, thereby contributing to the generation of locally amplified signals specifically at the leading edge, leading to actin polymerization and directional migration of leukocytes. →, promotion of protein-protein interactions or signal transduction; ⊣, inhibition of signal transduction.
Despite our initial findings on the importance of WDR26 and RACK1 in regulating leukocyte migration, a number of key questions remain unanswered. For example, it remains unknown what causes the membrane translocation and accumulation of WDR26 and RACK1 at the leading edge. Given that Gβγ forms a shallow gradient from leading to trailing edge in a polarized leukocyte, direct binding with Gβγ is unlikely to be sufficient for the accumulation of WDR26 and RACK1 at the leading edge. Nevertheless, our findings that pretreatment of cells with pertussis toxin to block Gi/o activation abolished WDR26 and RACK1 translocation suggest that active Gαi/o and/or Gβγ signaling is required for translocation.31,32 However, it remains to be delineated what downstream signaling pathways of Gβγ dictates translocation, and whether active Gαi/o signaling contributes to translocation.
Moreover, we do not know how WDR26 and RACK1 work in conjunction to balance the activation and inhibition of Gβγ signaling. Our data suggests that the interaction of RACK1 with Gβγ is subsequent to WDR26 binding to Gβγ, but we do not know about the dynamics of their interactions, nor do we know what regulates their alternate interactions with Gβγ.32 Based on our co-immunoprecipitation data, we find that WDR26 and RACK1 interact with Gβγ within 5 and 30 min of stimulation, respectively. However, the slow dynamics of complex formation likely reflects the lack of sensitivity of the assay rather than the actual dynamics in vivo, because membrane translocation and co-localization of WDR26 and RACK1 with Gβγ can be detected within one minute of stimulation, as revealed by immunofluorescence staining. More sensitive assays such as fluorescence resonance energy transfer (FRET) based approaches are required to further discern the exact dynamics of their interactions.
In addition to PI3K and PLCβ, additional Gβγ effectors may contribute to leukocyte migration, including PLA2, p38 MAPK, pREX1, Radil and mTORC2.15-20 It remains to be seen if WDR26 and RACK1 influence Gβγ-mediated activation of these effectors, and whether this regulation contributes to directional migration in leukocytes.
Finally, the molecular basis for the different functions of WDR26 and RACK1 in regulating Gβγ signaling is not clear. Crystal structure determination has shown that like Gβ, RACK1 adopts a seven-bladed β-propeller structure.33However, it has a short N-terminus (~10 aa) and does not require a Gγ-like protein to stabilize its structure.33 WDR26 shares little sequence homology with either RACK1 or Gβ, and is a much larger protein (~75 kDa vs. 36 kDa for RACK1 or Gβ). Based on sequence alignments with Gβ and other WD40 repeat proteins, WDR26 is predicted to contain six to seven WD40 repeat motifs at its C-terminus.32 However, it has an extensive N-terminus (~352 aa) that contains two distinguishable domains, Lis-homology (LisH) and C-terminal to LisH (CTLH) domains, which are implicated in protein-protein interactions and protein dimerization. Our studies have shown that RACK1 and WDR26 have distinct binding sites on Gβγ. While the RACK1-binding sites are located on one side-surface of Gβγ that has little overlapping with the Gα-contact surface, the WDR26-binding sites are contained in the region of Gβγ that interacts with Gα.30-32 Intriguingly, although the Gα contact surface of Gβγ is required for the activation of many Gβγ-effectors, the binding of WDR26 to Gβγ enhances rather than inhibits effector activation. The exact mechanisms for the action of WDR26 is not yet clear, but may be related to its ability to interact with both Gβγ and its effectors. By forming a complex with Gβγ and its effectors, WDR26 may force Gβγ to use alternative residues for more efficient activation of its effectors. Such a mechanism has been reported for one Gβγ-interacting protein, AGS8, which directs Gβγ to activate PLCβ2 through alternative activation sites.34 Alternatively, WDR26 may interact sequentially with Gβγ and its effectors to bring them in close proximity, thereby facilitating more efficient activation. Gβγ has been shown to bind to the N-terminal domain of RACK1 that comprise the first two WD40 repeats, whereas the binding sites of Gβγ on WDR26 have been mapped to a much more extensive region that are located in the C-terminal fragment of WDR26 between the LisH and the WD40 domains. Further work will be needed to discern the precise sites of WDR26 and RACK1 interactions with Gβγ and identify the structural determinants that are responsible for the different function of WDR26 and RACK1 in regulating Gβγ signaling.
In addition to regulating leukocyte migration, recent work has shown that Gβγ also plays a critical role in transmitting signals from multiple GPCRs to promote tumor growth and metastasis.35,36 Conceivably, aberrant regulation of Gβγ signaling by WDR26 and RACK1 may contribute to tumor progression. Indeed, our preliminary data show that WDR26 is overexpressed in human breast cancer tissue, and contributes to Gβγ-mediated tumor cell growth and migration. The role of RACK1 in promoting or suppressing tumor growth and metastasis has also been reported, but its functions in the context of Gβγ-mediated tumor progression remain unknown.37,38 Future work will be important to determine how altered expression of WDR26 and RACK1 results in aberrant Gβγ signaling and how this in turn contributes to various diseases.
Acknowledgments
The work was supported in part by National Institute of Health Grant GM094255 to S.C. and T32 pharmaceutical science and pain training grants to C.R.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/22940
References
- 1.Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys. 2010;39:265–89. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 3.Weiner OD. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol. 2002;14:196–202. doi: 10.1016/S0955-0674(02)00310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–47. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Wang F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb Perspect Biol. 2009;1:a002980. doi: 10.1101/cshperspect.a002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–14. doi: 10.1016/S0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 7.Chung CY, Potikyan G, Firtel RA. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol Cell. 2001;7:937–47. doi: 10.1016/S1097-2765(01)00247-7. [DOI] [PubMed] [Google Scholar]
- 8.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–23. doi: 10.1016/S0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 9.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–40. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–52. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, et al. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–27. doi: 10.1016/S0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 12.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/S0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 14.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HRA. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–13. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Das S, Losert W, Parent CA. mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev Cell. 2010;19:845–57. doi: 10.1016/j.devcel.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–14. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13:457–64. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W, Zhang Y, Xu W, Harden TK, Sondek J, Sun L, et al. A PLCβ/PI3Kγ-GSK3 signaling pathway regulates cofilin phosphatase slingshot2 and neutrophil polarization and chemotaxis. Dev Cell. 2011;21:1038–50. doi: 10.1016/j.devcel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Mo Z, Bokoch G, Guo C, Li Z, Wu D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr Biol. 2005;15:1874–9. doi: 10.1016/j.cub.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Welch HC, Condliffe AM, Milne LJ, Ferguson GJ, Hill K, Webb LM, et al. P-Rex1 regulates neutrophil function. Curr Biol. 2005;15:1867–73. doi: 10.1016/j.cub.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 21.Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Moutón C, Lacalle RA, Mira E, Jiménez-Baranda S, Barber DF, Carrera AC, et al. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–68. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–6. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- 24.Dell EJ, Connor J, Chen S, Stebbins EG, Skiba NP, Mochly-Rosen D, et al. The betagamma subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J Biol Chem. 2002;277:49888–95. doi: 10.1074/jbc.M202755200. [DOI] [PubMed] [Google Scholar]
- 25.Stirnimann CU, Petsalaki E, Russell RB, Müller CW. WD40 proteins propel cellular networks. Trends Biochem Sci. 2010;35:565–74. doi: 10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun Signal. 2011;9:22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol. 2006;8:1277–83. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- 28.Whiteway MS, Wu C, Leeuw T, Clark K, Fourest-Lieuvin A, Thomas DY, et al. Association of the yeast pheromone response G protein beta gamma subunits with the MAP kinase scaffold Ste5p. Science. 1995;269:1572–5. doi: 10.1126/science.7667635. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Dell EJ, Lin F, Sai J, Hamm HE. RACK1 regulates specific functions of Gbetagamma. J Biol Chem. 2004;279:17861–8. doi: 10.1074/jbc.M313727200. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Lin F, Hamm HE. RACK1 binds to a signal transfer region of G betagamma and inhibits phospholipase C beta2 activation. J Biol Chem. 2005;280:33445–52. doi: 10.1074/jbc.M505422200. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Lin F, Shin ME, Wang F, Shen L, Hamm HE. RACK1 regulates directional cell migration by acting on G betagamma at the interface with its effectors PLC beta and PI3K gamma. Mol Biol Cell. 2008;19:3909–22. doi: 10.1091/mbc.E08-04-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Z, Tang X, Lin F, Chen S. The WD40 repeat protein WDR26 binds Gβγ and promotes Gβγ-dependent signal transduction and leukocyte migration. J Biol Chem. 2011;286:43902–12. doi: 10.1074/jbc.M111.301382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz Carrillo D, Chandrasekaran R, Nilsson M, Cornvik T, Liew CW, Tan SM, et al. Structure of human Rack1 protein at a resolution of 2.45 Å. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:867–72. doi: 10.1107/S1744309112027480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan C, Sato M, Lanier SM, Smrcka AV. Signaling by a non-dissociated complex of G protein βγ and α subunits stimulated by a receptor-independent activator of G protein signaling, AGS8. J Biol Chem. 2007;282:19938–47. doi: 10.1074/jbc.M700396200. [DOI] [PubMed] [Google Scholar]
- 35.Tang X, Sun Z, Runne C, Madsen J, Domann F, Henry M, et al. A critical role of Gbetagamma in tumorigenesis and metastasis of breast cancer. J Biol Chem. 2011;286:13244–54. doi: 10.1074/jbc.M110.206615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bookout AL, Finney AE, Guo R, Peppel K, Koch WJ, Daaka Y. Targeting Gbetagamma signaling to inhibit prostate tumor formation and growth. J Biol Chem. 2003;278:37569–73. doi: 10.1074/jbc.M306276200. [DOI] [PubMed] [Google Scholar]
- 37.Shi S, Deng YZ, Zhao JS, Ji XD, Shi J, Feng YX, et al. RACK1 promotes non-small-cell lung cancer tumorigenicity through activating sonic hedgehog signaling pathway. J Biol Chem. 2012;287:7845–58. doi: 10.1074/jbc.M111.315416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng YZ, Yao F, Li JJ, Mao ZF, Hu PT, Long LY, et al. RACK1 suppresses gastric tumorigenesis by stabilizing the β-catenin destruction complex. Gastroenterology. 2012;142:812–23, e15. doi: 10.1053/j.gastro.2011.12.046. [DOI] [PubMed] [Google Scholar]


