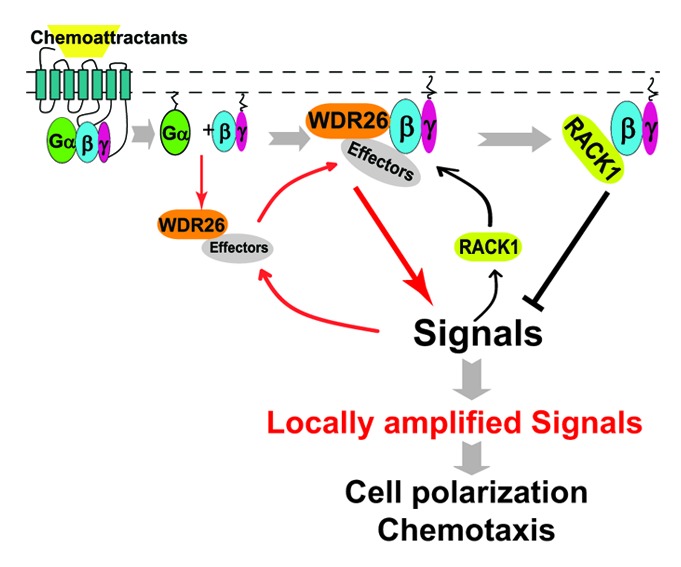
Figure 1. Working model for WDR26 and RACK1 mediated positive and negative regulation of Gβγ signaling at the cell’s leading edge during chemotaxis. In untreated cells, WDR26 binds select Gβγ effectors in the cytosol. Upon chemoattractant-mediated stimulation of Gi-coupled GPCRs, WDR26 and effectors are recruited to the membrane by Gαi/o and/or Gβγ-mediated signaling. Upon membrane recruitment, free Gβγ and WDR26 form a complex to promote Gβγ-mediated signal transduction, which may in turn lead to the recruitment of RACK1 from the cytosol. RACK1-binding to Gβγ inhibits Gβγ-mediated signal transduction by competing off either the Gβγ effectors, or the effectors in complex with WDR26. The combined activities of WDR26 and RACK1 serve to promote and fine tune Gβγ signaling, thereby contributing to the generation of locally amplified signals specifically at the leading edge, leading to actin polymerization and directional migration of leukocytes. →, promotion of protein-protein interactions or signal transduction; ⊣, inhibition of signal transduction.
