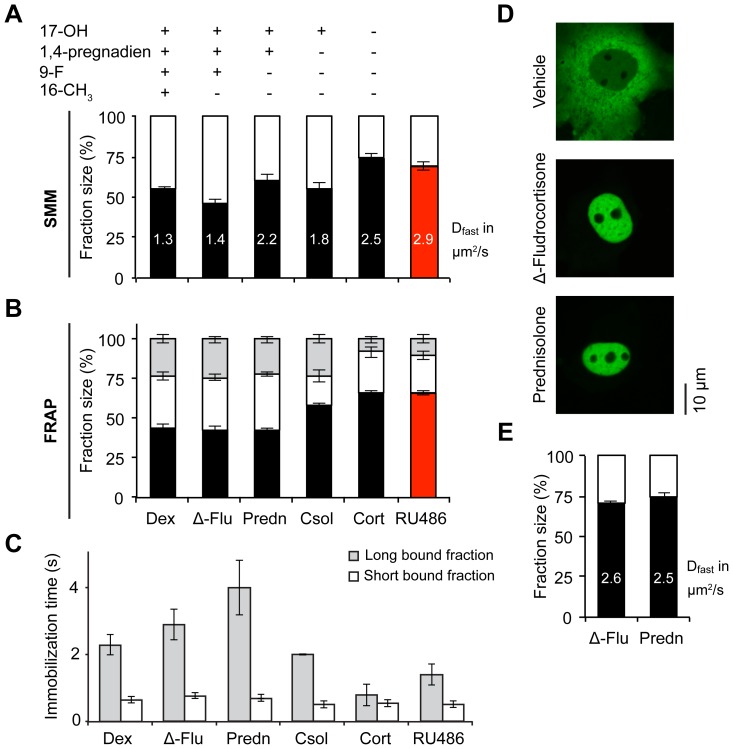
Figure 3. Ligand structure determines the nuclear mobility of the GR.
A range of natural and synthetic agonists (black bars) and an antagonists (red bar) were tested for their effect on the intranuclear mobility of the GR by both SMM (A) and FRAP (B–C) analysis. Multiple structural elements of the steroids are associated with a reduced mobility of the receptor. Altered mobility can be reflected in all aspects of mobility: a larger bound fraction (SMM; white bars and FRAP; white and light grey bars combined) a lower diffusion coefficient (in µm2/s, written in its corresponding bar in A) and longer immobilization times (C). (D and E) A mutation of phenylalanine 623 to alanine (F623A) prevents interactions of the 9-fluoro group of steroids within the ligand binding pocket of the GR. F623A YFP-GR still translocates completely to the nucleus after 3 hours of 1 µM prednisolone or Δ-fludrocortisone treatment (D). SMM analyses of nuclear F623A YFP-GR kinetics shows that the mobility of F623A YFP-GR is highly similar after either Δ-fludrocortisone or prednisolone treatment (black bars for the diffusing fraction, with their corresponding diffusion coefficient (in µm2/s) written within their corresponding bar; (E)). SMM: n = 20, FRAP: n = 30. Data represented as total fit ± SEM (of 3 separate PICS analyses) for SMM and as average of top 10% fits ± SEM for FRAP. Δ-flu; Δ-fludrocortisone, dex; dexamethasone, Predn; prednisolone, csol; cortisol, cort; corticosterone. The data for GR-dexamethasone is the same as in Figure 2.