Figure 4. Ligand structure determines the nuclear mobility of the MR.
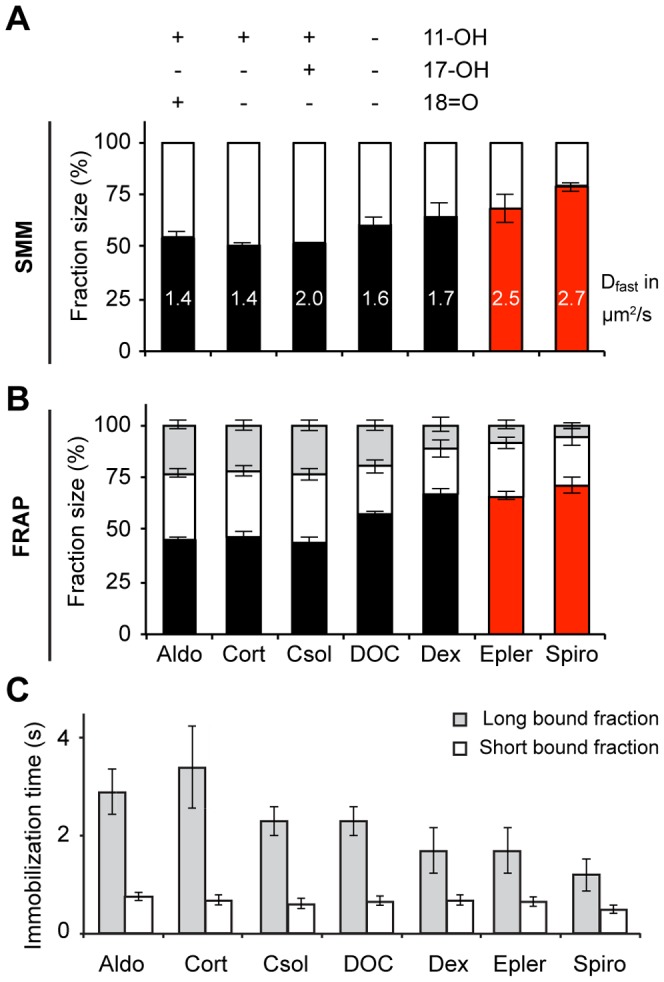
A range of natural and synthetic agonists (black bars) and antagonists (red bars) were tested for their effect on the intranuclear mobility of the MR by both SMM (A) and FRAP (B–C) analysis. The MR and GR share several agonists, but the binding and functional characteristics differ. Indeed, a weak agonist for the GR, corticosterone (cort), which gave a very mobile GR, instead leads to a low mobility for the MR. A large bound fraction (SMM; white bars and FRAP; white and light grey bars combined) a low diffusion coefficient (in µm2/s, written within its corresponding bar in A) and long immobilization times (C). Of all functional steroid side groups, only the 18-keto (18 = O) group appears to affect the mobility of the MR. SMM: n = 20, FRAP: n = 30. Data represented as total fit ± SEM (of 3 separate PICS analyses) for SMM and as average of top 10% fits ± SEM for FRAP. Aldo; aldosterone, csol; cortisol, DOC; deoxycorticosterone, dex; dexamethasone, epler; eplerenone, spiro; spironolactone.
