Abstract
Heightened impulsivity is a feature of some psychiatric disorders, including addiction, that also have sex-specific patterns of expression. The relationship between addiction and impulsivity may be driven by drug-induced changes in behavior caused by long term adaptations in signaling within the medial prefrontal cortex (mPFC). Here, we used a response inhibition task that is sensitive to changes in mPFC function to examine the effects of sex and exposure to amphetamine (AMPH) on impulsive action and vigilance. We also examined drug-induced alterations in glutamatergic and dopaminergic signaling through challenge injections with the NMDA receptor antagonist MK-801 (dizocilpine) and AMPH. Male and female Sprague Dawley rats were injected (i.p.) with saline or 3 mg/kg AMPH every other day during adolescence (postnatal day (P) 27–45) or adulthood (P85–103). Starting on P125–135, rats were tested for their ability to lever press for a food reward during periods of signaled availability and withhold responding during a “premature response” phase. In experiment 1, rats received challenge injections (i.p.) of MK-801 and AMPH followed by tests of task performance and locomotor activity. In experiment 2, rats received intra-mPFC infusion of MK-801. We found that females had better inhibitory control and poorer vigilance than males and that AMPH exposure had both sex- and age-of-exposure dependent effects on impulsivity. Systemic drug challenges disrupted task performance, particularly in females, and increased impulsivity while intra-mPFC infusions had modest effects. AMPH exposure did not affect responses to drug challenges. Together, these results suggest that sex mediates both trait and drug-induced impulsivity.
Keywords: sex differences, amphetamine, rat, impulsivity, adolescence, glutamate
1. Introduction
Impulsive behavior is frequently associated with psychopathology, including disorders such as attention deficit-hyperactivity disorder (ADHD), obsessive-compulsive disorder, schizophrenia and substance abuse [1–5]. Drug abuse is associated with elevated levels of impulsivity [6–8]; for example, both recreational and dependent stimulant users are impaired in measures of response inhibition [9–11]. Interestingly, sex differences in the incidence and presentation of these disorders sometimes co-vary with impulsivity [12,13]. Fewer females are diagnosed with ADHD and those diagnosed are less impulsive than their male counterparts [12,14,15]. Females with schizophrenia are diagnosed later in life compared to males [16], but they exhibit more “positive” symptoms including increased impulsivity [17,18]. Healthy females tend to be less impulsive, though few human studies that specifically address this are available at this time [19]. Sex differences in impulsivity, if present, could explain sex differences in the development of some psychopathologies.
Animal studies provide evidence for sex differences in impulsivity, but the direction of these differences has depended on the type of impulse control under investigation. Compared to males, female rats are more impulsive on tests of impulsive choice [20], but they appear less impulsive on tests of impulsive action [21,22]. One caveat of the studies reporting decreased impulsive action in females is that they have often relied on tasks that are primarily designed to test attention, including the five choice serial reaction time (5-CSRT) task [22] and the spatial divided attention task [21]. Not only do males exhibit better attention during task performance [21], but they also exhibit heightened vigilance (i.e., sustained attention) during long delays [22]. Because attention and vigilance are difficult to isolate from impulsive action, tasks requiring significant attentional demands may complicate the identification of sex differences in impulsivity.
The present study was designed to investigate the potential interaction of sex and drug exposure history on impulsive action using a task with relatively low attentional demands [23]. We have previously demonstrated that there are greater deficits in working memory [24] and behavioral flexibility [25] when rats are exposed to amphetamine (AMPH) during adolescence compared to adulthood. In addition, we have found increased impulsive-like behavior following adolescent AMPH exposure [26]. These studies were all done in males, however. Female rats are known to be more sensitive to the acute effects of AMPH [27,28] and AMPH-induced locomotor sensitization [29,30], which may also lead them to be more sensitive to the effects of AMPH on impulsivity. Moreover, the influence of sex on sensitization may interact with age, as adolescent females exhibit enhanced AMPH sensitization relative to adult females and adolescent males [31].
A secondary goal of the present study was to determine the extent to which differences in glutamate receptor function might contribute to group differences in impulsive action. Withdrawal from AMPH exposure is associated with reduced expression of NMDA and AMPA receptors in the mPFC [32,33] and performance on the response inhibition task utilized in the current experiment has previously been associated with glutamate receptor plasticity in the prelimbic region of the mPFC [23]. We therefore hypothesized that AMPH-exposure would influence the response to an NMDA antagonist, MK-801, known to increase impulsive action [34].
2. Methods
2.1. Subjects
A total of 150 male and female Sprague-Dawley rats, which were born in our animal facility from breeders originally obtained from Harlan (Indianapolis, IN, USA), were used in these experiments (n = 72 and 78 for experiments 1 and 2, respectively). In experiment 1, group sizes were 11–13 rats/treatment condition/sex. In experiment 2, group sizes were initially 12–14 rats/treatment/sex, but final group sizes were 10–13/treatment/sex. Nine rats either did not complete testing or were excluded from the final analysis due to technical difficulties including health complications, headpiece malfunction, or inaccurate cannulae placement. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana-Champaign, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Rats were weaned on postnatal day (P) 22 and housed with same-sex littermates in groups of 2–3 per cage for the duration of the experiment (experiment 1) or until they underwent cannulation surgery (experiment 2). Rats were kept on a 12-h light/dark cycle (lights on at 0800 h) in a temperature-controlled room and water was available ad libitum throughout the study. Food was available ad libitum until either P120 (experiment 1) or 5 days after surgery (P125–130; experiment 2). At this time, rats were restricted to 6 g/day (females) or 8 g/day (males). Food allotments were adjusted individually in 1–2 g increments so that rats were maintained at 85–90% of free-feeding weight for the duration of instrumental behavior training and testing. Behavioral training and testing was performed Sunday through Friday between 1000 and 1600 h. Locomotor testing occurred between 1600 and 2000 h.
2.2. Drugs
d-AMPH sulfate (Sigma, St. Louis, MO, USA) and MK-801 maleate (dizocilpine; Abcam Inc., Cambridge, MA, USA) were dissolved in saline, with doses calculated as the weight of the salt. Intraperitoneal (i.p.) injections were given at a volume of 1ml/kg. In Exp. 2, frozen aliquots of vehicle and MK-801 were allowed to defrost and come to room temperature before they were infused.
2.3. Apparatus
Response inhibition training was conducted in standard operant chambers (Coulbourn Instruments; Allentown, PA, US), located within sound attenuating cubicles. Chambers were equipped with a food trough in the center of the front wall. The food trough contained a white cue light and an infrared photocell that detected head entries. Retractable levers were located to the left and right of the food trough; a white cue light was located directly above each lever. Rats were randomly assigned to use either the left or the right lever at the beginning of behavioral training; the inactive lever remained retracted throughout the experiment. A white houselight was located near the chamber ceiling on the back wall. Sessions were recorded and analyzed using Graphic State software (v3.1; Coulbourn Instruments).
Locomotor activity was measured in an open-field arena that consisted of an acrylic box (40.6 × 40.6 × 40.6 cm) surrounded by a photobeam frame (Coulbourn Instruments). The arena was housed in a sound attenuating chamber (76 × 80 × 63 cm) that had two ceiling mounted white lights (4W each) and a 76-mm speaker located on one inside wall. The speaker was connected to a white noise generator (Coulbourn Instruments) that was active during testing (70 dB). Computer software (TruScan v 2.01, Coulbourn Instruments) was used to record photobeam breaks (100 ms sampling rate) and convert this to measures of ambulatory distance (m) or stereotypy (see below).
2.4. Saline or AMPH pre-exposure
Rats in both experiments received 10 i.p. injections (1ml/kg), every other day, during both adolescence (P27–45) and adulthood (P85–103). Rats in the adolescent-exposed group received 3.0 mg/kg d-AMPH sulfate during adolescence and 0.9% saline during adulthood. Rats in the adult-exposed group received 0.9% saline during adolescence and 3.0 mg/kg d-AMPH sulfate during adulthood. Control animals received 0.9% saline during both adolescence and adulthood. Rats were injected in a separate room and placed into individual cages lined with hardwood bedding. After 60 min, they were returned to their home cages and moved back to the colony room.
2.5. Experiment 1
2.5.1. Instrumental Training
On the fifth day of food restriction (P125), rats were given 10 of the reinforcements used in this study (45 mg pellets; Bioserv, #F0021; Frenchtown, NJ) in their homecage. On the next day, rats underwent magazine training wherein a total of 20 food pellets were delivered on a variable time 90-s (60–120 s) schedule. During pellet delivery, the house light was extinguished and a cue light within the food trough was illuminated for 1 s. The following day, rats began daily lever press training sessions. During these sessions, the house light was extinguished, a single lever was extended, and the cue light above the lever was illuminated. A lever press resulted in lever retraction, extinguishing of the lever cue light, delivery of a food pellet, and a 1-s illumination of the food trough cue light. Sessions lasted for 60 min or until 150 pellets were earned. If a rat obtained fewer than 10 pellets during a lever press training session, that session was followed by a session of hand shaping. Lever press training continued until rats met a criterion of ≥ 80 reinforcements/session for two consecutive sessions.
2.5.2. Response inhibition training
After they met the initial training criterion, rats received 2–3 daily sessions of a “no delay” version of the response inhibition task (adapted from Hayton, Lovett-Barron, Dumont, & Olmstead, 2010). These sessions began with a 10-s intertrial interval (ITI) where all lights were extinguished and both levers were retracted. Each subsequent trial had a 5-s “reinforced phase” where the lever was extended, the cue light above the lever was illuminated, and a response led to the delivery of a food pellet. As before, pellet delivery was associated with lever retraction, extinguishing of the cue light above the lever, and illumination of the food trough cue light for 1 s. If the rat did not respond during the reinforced phase the trial was scored as an omission and the next ITI began. Each session consisted of 150 trials.
Rats were next trained on the delay version of the response inhibition task, which required them to withhold responding until the passage of a “premature phase” that was either 0.5, 1, or 2 s in duration. This phase occurred at the start of each trial and was signaled by illumination of the house light and extension of the lever. Responses during this phase, which were scored as premature, ended the trial and began the 10-s ITI. If rats withheld responding during this phase, they progressed to the reinforced phase, as described above. Each session consisted of 150 trials, with 50 trials each of the three premature phase delays presented in random order. Training continued until rats met a stability criterion of two consecutive sessions with < 10% variation in inhibitory control, which was calculated by the following equation [(reinforced responses)/(reinforced responses + premature responses)].
2.5.3. Effects of systemic drug challenges
After reaching the stability criterion, rats (n = 38 male, 34 female) were given a series of drug challenges to determine the effect on response inhibition task performance. First, they were habituated to the testing procedure with a single session in the task 30-min after they were injected with saline (1 ml/kg, i.p.). Next, they were tested with MK-801 (0.03, 0.06, or 0.08 mg/kg) and AMPH (0.3, 0.6, 0.9 mg/kg) in four-day blocks. On day 1 of each block, all rats were given saline. On days 2–4, rats were given drug doses in random order (using a modified Latin Square Design). MK-801 was tested during the first four-day block and was given 30-min prior to the start of the task; AMPH was tested second and was given 15 min prior to task start.
Following the test with their last dose of AMPH, rats were given ad libitum access to food in their home cages and remained in their home colony for 8–10 days. They were then tested for their locomotor response to a challenge does of AMPH (1.0 or 3.0 mg/kg, i.p.) during two sessions that were separated by 8–10 days. During these tests, rats were placed in an open-field arena for 30 min, injected (i.p.) with saline and returned to the open-field for 30 min, and were then given AMPH. Rats were allowed to behave undisturbed in the open-field for 60 min following the AMPH injection.
2.6. Experiment 2
2.6.1. Surgery
Between P120 and P125, rats in experiment 2 underwent surgery to implant a bilateral guide cannula (Part # C235G-1.5/SPC; Plastics One, Roanoke, VA) targeted at the prelimbic region of the mPFC. Briefly, rats were anesthetized with ketamine and xylazine (90 and 10 mg/kg, respectively; i.m.) and placed in a stereotaxic frame. Holes were drilled in the skull overlying the mPFC (+3.0 mm from bregma and ±0.75 mm from the midline; Paxinos and Watson, 2006) and the guide was lowered 3.2 mm below the skull surface. Four additional holes were drilled in the skull for insertion of stainless-steel support screws and dental acrylic was added to cement the cannula in place. A dummy cannula cut to the same length as the guide was then inserted to prevent obstruction. Rats were allowed to recover for at least five days before food restriction was initiated.
2.6.2. Effects of MK-801 infusion in the mPFC
On the fifth day of food restriction (P130–P135), rats underwent instrumental and response inhibition training, as described for Experiment 1. After they met the response inhibition stability criterion, rats were habituated to the infusion test procedure via a “mock” infusion: they were briefly restrained, the dust cap overlying the guide cannulae was removed, the infusion pump was operated for 1-min, and the dust cap was replaced after 2 additional min elapsed. Rats remained in their home cages for 5-min before starting the response inhibition session. Prior to the start of the next three daily response inhibition sessions, rats were given bilateral infusions of saline or MK-801 (2.0 or 4.0 μg/side), with MK-801 doses presented in random order before the last two test sessions. During infusion, the dust cap and obdurators were removed, two 4.2 mm injectors (Part # C235I/SPC; Plastics One, Roanoke, VA) were inserted into the guide cannulae, and 0.5 μl was infused over a 1-min period. Injectors remained in place for 2-min to allow for diffusion. The injectors were then removed, the obdurators and dustcap were replaced, and the rat was returned to its home cage for 5-min.
2.6.3. Histology
At the completion of behavioral testing, rats were deeply anesthetized with chloral hydrate (800 mg/kg, i.p.) and neutral red (0.5 μl/side) was infused into the guide cannulae to aid in identification of the injection site. Rats were then perfused transcardially with saline (60 mL) followed by buffered formalin (60 mL). Their brains were then removed and stored in formalin for at least seven days before they were sectioned (60 μm slices) on a freezing stage microtome. The series of sections encompassing the guide cannulae and injector tip was stained with cresyl violet and examined under a light microscope to verify infusion locations. Rats were excluded from data analysis if either side of the bilateral cannulae did not terminate in the prelimbic region of the mPFC (Fig. 6A).
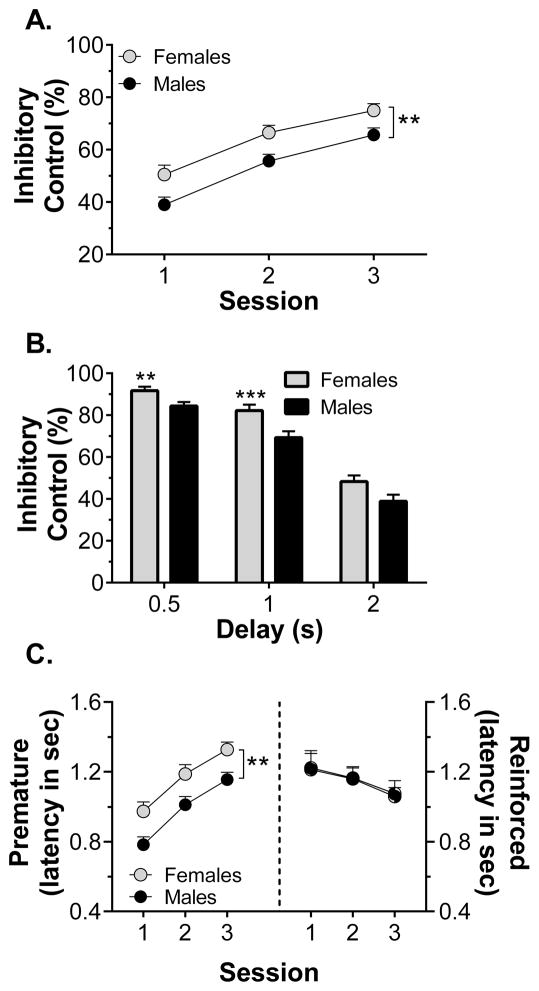
Figure 6.
The effect of sex on performance during the first three response inhibition training sessions in experiment 2 (n = 38–40/group). Panel A shows sex differences in inhibitory control across the three sessions, collapsed across treatment group. Panel B shows sex differences, collapsed across treatment group, in latency to make a premature (left) or reinforced (right) lever press. Panel C shows sex differences in inhibitory control, collapsed across treatment and session, for each of the premature delay durations. ** p < 0.01, *** p < 0.001 vs males
2.7. Data analysis
In both experiments, the number of responses, the latency to make these responses, and the number of omissions were recorded for each response inhibition session; responses were either premature or reinforced in sessions with a delay. Inhibitory control was calculated for each session with a delay by dividing the number of reinforced responses by the total number of responses for each of the three premature delay lengths. This inhibitory control measure was then analyzed using a four-way ANOVA; sex and treatment were the between-subjects factors while premature delay length and session (or dose) were the within-subjects factors. In addition, inhibitory control during the criterion session was analyzed with a three-way ANOVA, with sex and treatment as between-subjects factors and delay length as the within-subjects factor.
Latency was calculated for each session by taking the average latency to make a premature or reinforced response. For “no-delay” sessions, all responses were reinforced. Because many rats made no premature responses during the 0.5 and 1-s premature delay lengths, only trials with a 2-s premature delay length were used to calculate latency in delay sessions. Latency and the number of omissions were then analyzed using a three-way ANOVA with sex and treatment as between-subjects factors and session (or dose) as a within-subjects factor. In addition, latency during the criterion session, as well as the number of sessions required to reach criterion, were analyzed using a two-way ANOVA with sex and treatment as between-subjects factors.
For each of the open-field tests, activity was quantified during the 60 min following AMPH injection via software that provided a measure of ambulation and the total number of stereotyped movements. Ambulation was calculated by tabulating consecutive photobeam breaks in the horizontal plane and converting this to distance (in meters). Stereotypy, which is a measure of repetitive behavior such as head and body swaying, head bobbing, and sniffing, was calculated by tabulating repetitive beam breaks in a focused area that do not contribute to large changes in location in the open-field. Ambulation and stereotypy were then analyzed using separate three-way ANOVAs with sex and treatment as between-subjects factors and AMPH dose as the within-subjects factor.
Because females differ in their response to AMPH during both adolescence [31], and adulthood [29,30], the effects of AMPH exposure were also examined separately in males and females. Planned three-way ANOVA were run within each sex for all previously mentioned four-way ANOVAs, with treatment as the between-subjects factor and premature delay length and session (or dose) as the within-subjects factors. All tests were performed in SAS (version 9.3). Alpha level was set to p < 0.05 and Tukey HSD post-hoc tests were used to investigate significant main effects and interactions. Because the residuals of the number of omissions in the response inhibition task were not normally distributed, a power transformation was performed on these data before they were analyzed statistically.
3. Results
3.1. Experiment 1
3.1.2. Response Inhibition training
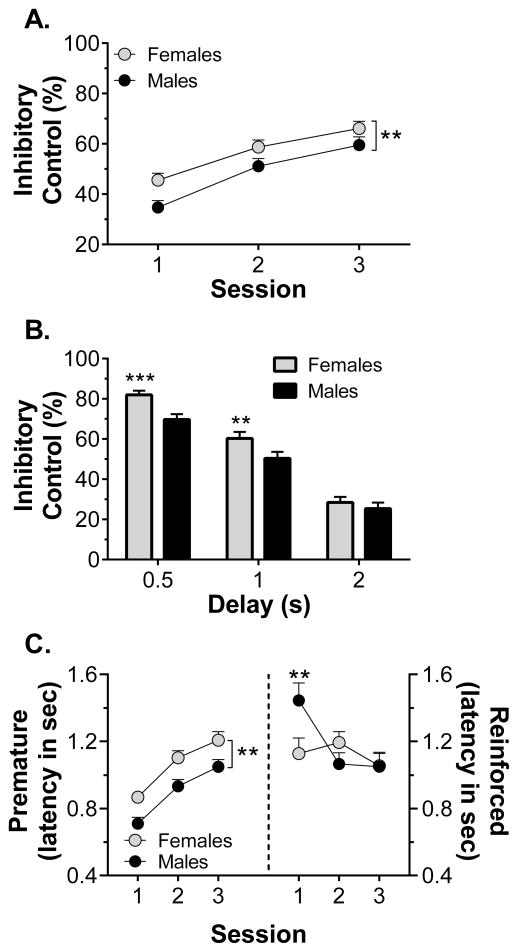
Inhibitory control improved across the first three delay training sessions. Four-way ANOVA revealed significant main effects of session (F2,132 = 48.2, p < 0.001), delay (F2,132 = 964.8, p < 0.001) and sex (F1,66 = 7.50, p = 0.008), but no main effect of treatment. As shown in Fig. 1A with the data collapsed across treatment group, females exhibited greater inhibitory control across sessions compared to males. There also were sex x delay (F2,132 = 48.2, p < 0.001) and session x delay (F2,132 = 48.2, p < 0.001) interactions. Sex differences in inhibitory control were only evident at the 0.5 and 1-s delays, as shown in Fig. 1B, with data collapsed across treatment group. There were no significant effects of sex or treatment on the number of sessions required to meet the stability criterion (i.e., two consecutive sessions with < 10% variation in inhibitory control). When the data were collapsed across group, the mean sessions to criterion was 5.62 ± 0.43 and 6.33 ± 0.41 sessions for females and males, respectively. However, during the criterion session, females were still significantly less impulsive than males (83.2 ± 2.5% and 75.1 ± 2.4%, respectively, collapsed across treatment). Three-way ANOVA revealed significant main effects of sex (F1,66 = 5.31, p = 0.024) and delay (F2,132 = 159.2, p < 0.001), but no main effect of treatment and no interactions.
Figure 1.
The effect of sex on performance during the first three response inhibition training sessions in experiment 1 (n = 34–38/group). Panel A shows sex differences in inhibitory control across the three sessions, collapsed across treatment group. Panel B shows sex differences, collapsed across treatment group, in latency to make a premature (left) or reinforced (right) lever press across the three sessions. Panel C shows sex differences in inhibitory control, collapsed across treatment and session, for each of the premature delay durations. ** p < 0.01, *** p < 0.001 vs males
Measures of latency to make a lever press response during the premature or reinforced phases of each trial also revealed evidence for sex differences. Females were slower to respond during no-delay training, relative to males. Three-way ANOVA of response latency revealed main effects of sex (F1,66 = 9.32, p = 0.003) and session (F1,66 = 161, p < 0.001). Response times during no-delay training for females and males were 1.72 ± 0.06 sec and 1.46 ± 0.06 sec, respectively, collapsed across treatment and session. During the first three delay training sessions, latency to make a premature response increased and three-way ANOVA revealed significant main effects of session (F2,132 = 74.1, p < 0.001) and sex (F1,66 = 8.64, p = 0.005). There was no main effect of treatment and no interactions. With data collapsed across treatment groups (Fig. 1C), it is evident that males made premature responses more quickly than females. The statistical significance of this difference was verified by significant main effects of sex (F1,66 = 8.64, p = 0.005) and session (F2,132 = 74.1, p < 0.001). During the criterion session, two-way ANOVA of premature response latency revealed that the main effect of sex persisted (F1,66 = 4.17, p = 0.045), with females and males making premature responses after 1.43 ± 0.05 sec and 1.29 ± 0.05 sec, respectively (collapsed across treatment). In contrast to this sex difference in premature response latency, the latency to make a reinforced response (Fig. 1C) was not different across treatment, sex or training sessions. There were also no effects of treatment or sex on reinforced response latency during the criterion session, where females responded in 1.03 ± 0.06 sec and males responded in 1.04 ± 0.06 sec (collapsed across treatment).
Planned three-way ANOVA of inhibitory control within each sex revealed main effects of session (F2,62 = 100.0, p < 0.001) and delay (F2,62 = 93.0, p < 0.001), as well as treatment x session (F4,62 = 3.62, p = 0.010) and session x delay (F4,124 = 3.40, p = 0.011) interactions in females. The treatment x session interaction was due primarily to the relatively low inhibitory control exhibited by adult-exposed females during the first session (Fig. 2A). In males the planned three-way ANOVA revealed main effects of session (F2,70 = 136.9, p < 0.001) and delay (F2,70 = 81.5, p < 0.001), but no effect of treatment and no interactions (Fig. 2B).
Figure 2.
The effect of amphetamine exposure on performance during the first three response inhibition training sessions in experiment 1 (n = 11–13/group). Panels A and B show the effect of treatment on inhibitory control across the three sessions in females and males, respectively. # p <0.05 vs adolescent-exposed females
3.1.3 Effects of systemic drug challenges
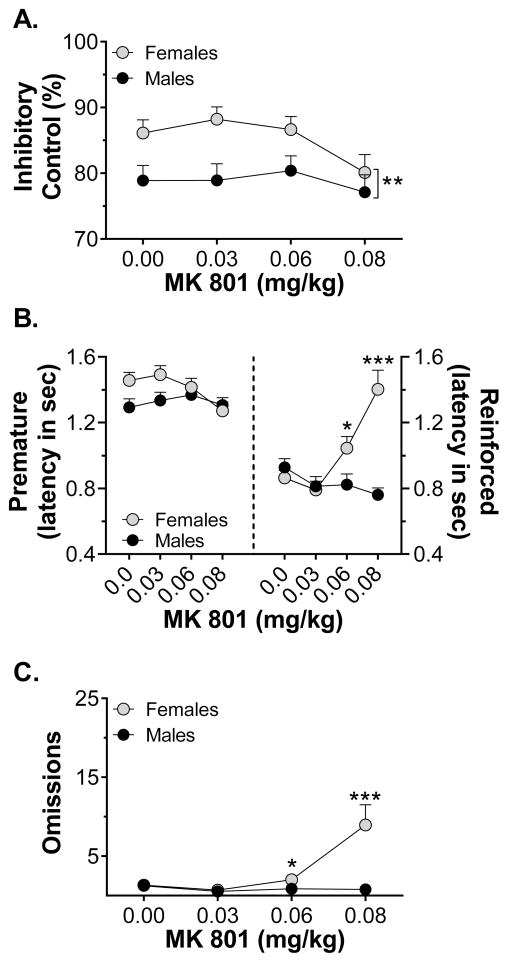
Challenge injections of MK-801 reduced inhibitory control, but only in females. Four-way ANOVA revealed a main effect of sex (F1,66 = 4.67, p = 0.034), but the main effect of dose failed to reach significance (p = 0.082) and there was no effect of treatment and no interactions. While females had better inhibitory control, they also appeared to be more sensitive than males to the effect of MK-801 (Fig. 3A). Planned three-way ANVOA revealed main effects of dose (F3,93 = 11.8, p < 0.001) and delay (F2,62 = 43.8, p < 0.001) in females, while dose failed to reach significance in males (p = 0.055). MK-801 also reduced latency to make a premature response: three-way ANOVA revealed a main effect of dose (F3,198 = 4.62, p = 0.004), but the sex x dose interaction failed to reach significance (p = 0.087). MK-801 increased latency to make a reinforced response in females only, as shown in Fig. 3B, with data collapsed across treatment group. Three-way ANOVA revealed main effects of dose (F3,198 = 7.38, p < 0.001) and sex (F1,66 = 8.18, p = 0.006), as well as a sex x dose interaction (F3,198 = 12.4, p < 0.001), but no effect of treatment and no interactions. Finally, MK-801 increased omissions; three-way ANOVA of the transformed omission data revealed main effects of dose (F3,198 = 4.27, p = 0.006) and sex (F1,66 = 11.7, p = 0.001), as well as a sex x dose interaction (F3,198 = 8.03, p < 0.001), but no effect of treatment and no interactions. Females made more omissions than males at the highest dose of MK-801, as shown in Fig. 3C, with data collapsed across treatment.
Figure 3.
Sex differences in the response to systemic MK-801 injection, collapsed across treatment group (n = 34–38/group). Panel A shows inhibitory control following MK-801. Panel B shows latency to lever press during the premature (left) and response (right) phases. Panel C shows the number of trials omitted in each session. * p < 0.05, ** p < 0.01, *** p < 0.001 vs males
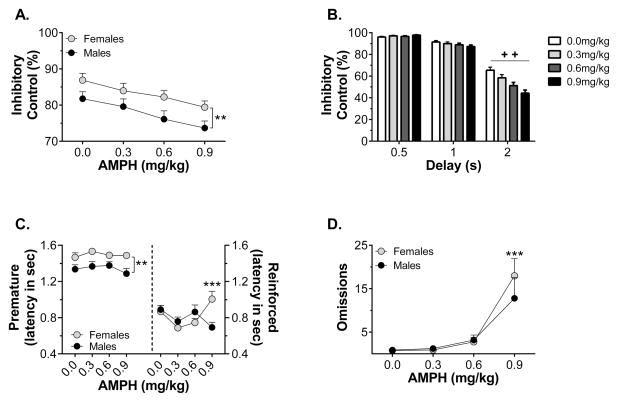
Challenge injections of AMPH reduced inhibitory control more robustly than challenge injections of MK-801. Four-way ANOVA revealed main effects of dose (F3,197 = 5.78, p < 0.001), sex (F1,66 = 11.8, p = 0.001), and delay (F2,132 = 882.8, p < 0.001), but no effect of treatment. Females displayed better inhibitory control across doses of AMPH, as shown in Fig. 4A with data collapsed across treatment. The four-way ANOVA also revealed sex x delay (F2,132 = 6.54, p = 0.002) and dose x delay (F6,394 = 9.57, p < 0.001) interactions. Examination of the dose x delay interaction revealed that AMPH challenge reduced inhibitory control only during 2-s delay trials, as shown in Fig. 4B with data collapsed across sex and treatment group. However, premature response latency during these trials was unaffected by AMPH dose. Three-way ANOVA revealed only a main effect of sex (F1,66 = 7.79, p = 0.007); females made premature responses more slowly, as shown in Fig. 4C, collapsed across treatment. Reinforced response latency, however, was increased following AMPH challenge. Three-way ANOVA revealed a main effect of dose (F3,198 = 3.73, p = 0.012) and a sex x dose interaction (F3,198 = 7.01, p < 0.001), but no effect of treatment. As shown in Fig. 4C, with data collapsed across treatment, females took longer to make a reinforced response at the highest dose of AMPH. AMPH also increased the number of omissions. Three-way ANOVA of the transformed omission data revealed a main effect of dose (F3,198 = 11.4, p < 0.001) and a sex x dose interaction (F3,198 = 4.13, p = 0.007). Females made more omissions than males at the highest dose of AMPH, as shown in Fig. 4D, with data collapsed across treatment.
Figure 4.
Sex differences in the response to systemic AMPH injection and the effect of delay duration on the response to AMPH. Panel A shows inhibitory control following AMPH, collapsed across treatment group (n = 34–38/group). Panel B shows latency to lever press during the premature (left) and response (right) phases, collapsed across treatment group (n = 34–38/group). Panel C shows the number of trials omitted in each session, collapsed across treatment group (n = 34–38/group). Panel D shows the effect of AMPH on inhibitory control at each of the premature delay durations, collapsed across sex and treatment group (n = 72). ** p < 0.01, *** p < 0.001 vs males; + + all dose comparisons significant at p < 0.01 for this delay
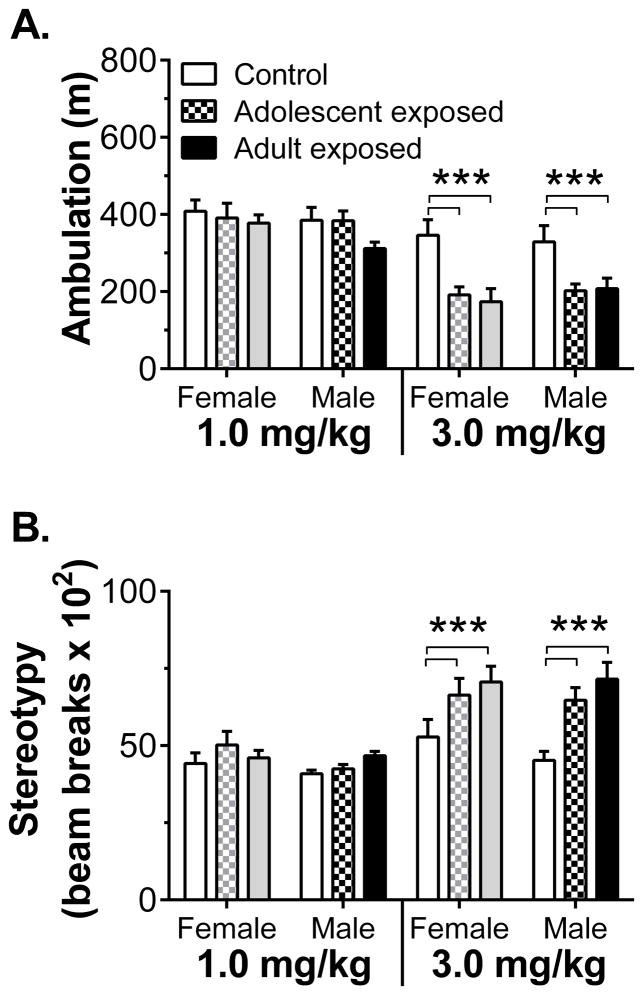
The locomotor response to subsequent challenge with 1.0 or 3.0 mg/kg AMPH revealed evidence for lasting sensitization in rats pre-exposed to the drug (Fig. 4). Three-way ANOVA of ambulation in the open-field revealed main effects of dose (F1,65 = 104, p < 0.001) and treatment (F2,65 = 8.66, p < 0.001), as well as a treatment x dose interaction (F2,65 = 8.79, p < 0.001). Post hoc analysis revealed that, relative to controls, rats exposed to AMPH during adolescence or adulthood had significantly lower levels of ambulation following injection of 3.0 mg/kg AMPH (Fig. 5A). This reduction in ambulation was due to an increase in a competing behavior, stereotypy. Three-way ANOVA of stereotypy revealed main effects of dose (F1,65 = 61.7, p < 0.001) and treatment (F2,65 = 10.7, p < 0.001), as well as a treatment x dose interaction (F2,65 = 6.31, p = 0.003). Post hoc analysis revealed that, relative to controls, adolescent- and adult-exposed rats had significantly higher levels of stereotypy following 3.0 mg/kg AMPH (Fig. 5B).
Figure 5.
Activity during 60-min period following 1.0 and 3.0 mg/kg AMPH injection (n = 11–13/group). Panel A shows cumulative ambulation (m). Panel B shows the cumulative number of stereotyped movements. *** p < 0.001 vs. control.
3.2. Experiment 2
3.2.1. Response Inhibition training
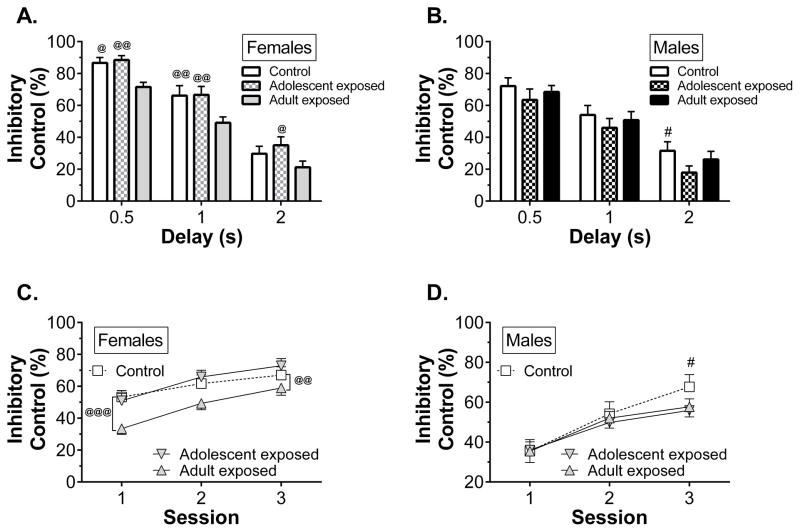
In experiment 2, inhibitory control improved across the first delay three training sessions. Four-way ANOVA revealed main effects of session (F2,144 = 40.7, p < 0.001), sex (F1,72 = 7.35, p = 0.008), and delay (F2,144 = 1000.7, p < 0.001). Females displayed better inhibitory control across sessions, as shown in Fig. 6A with data collapsed across treatment. Four-way ANOVA also revealed session x delay (F4,288 = 5.35, p < 0.001), sex x delay (F2,144 = 9.62, p < 0.001), sex x treatment (F2,72 = 3.30, p = 0.043), and sex x treatment x delay (F4,144 = 2.44, p = 0.049) interactions. Sex differences in inhibitory control were only evident at the 0.5 and 1-s delays, as shown in Fig. 6B, with data collapsed across treatment group. The sex x treatment and sex x treatment x delay interactions were explored through planned three-way ANOVA within each sex (see below). The number of sessions required to reach criterion (i.e., two consecutive sessions with < 10% variation in inhibitory control) depended on sex and treatment. Adolescent-exposed males required the greatest number of sessions to reach criterion – a mean of 8.75 sessions compared to means between 5.46 and 6.86 sessions for all other groups. Two-way ANOVA revealed a main effect of sex (F1,72 = 7.69, p = 0.007) and a sex x treatment interaction (F2,72 = 4.75, p = 0.012). Upon reaching criterion, the effects of sex and treatment on inhibitory control were no longer present, with females and males displaying similar levels of inhibitory control (77.9 ± 2.9% and 74.1 ± 2.9%, respectively, collapsed across treatment).
Similar to what we observed in Exp. 1, measures of latency to make a lever press response during the premature or reinforced phases of each trial also revealed evidence for sex differences. Females were slower to respond during no-delay training, relative to males. Three-way ANOVA of response latency revealed main effects of sex (F1,72 = 27.0, p < 0.001) and session (F1,72 = 276, p < 0.001); the effect of treatment failed to reach significance (p = 0.051). Response times during no-delay training for females and males were 1.68 ± 0.06 s and 1.27 ± 0.06 s, respectively, collapsed across treatment and session. During the first three delay training sessions, latency to make a premature response increased and three-way ANOVA revealed main effects of session (F2,144 = 64.5, p < 0.001) and sex (F1,72 = 11.3, p = 0.001), but no effect of treatment and no interactions. With data collapsed across treatment groups (Fig. 6C), it is evident that males made premature responses more quickly than females. During the criterion session, two-way ANOVA of premature response latency revealed that the main effect of sex persisted (F1,72 = 5.20, p = 0.026), with females and males making premature responses after 1.35 ± 0.05 s and 1.19 ± 0.05 s, respectively (collapsed across treatment). Latency to make a reinforced response decreased across the first three sessions. Three-way ANVOA revealed a main effect of session (F2,139 = 3.46, p = 0.034) as well as a sex x session interaction (F2,139 = 6.22, p = 0.003), but no effect of treatment and no interactions. With data collapsed across treatment groups (Fig. 6C), it is clear that females made reinforced responses more quickly during the first session. There were no effects of sex or treatment on latency to make a reinforced response during the criterion session, where females responded in 1.00 ± 0.06 s and males responded in 1.05 ± 0.07 s (collapsed across treatment)
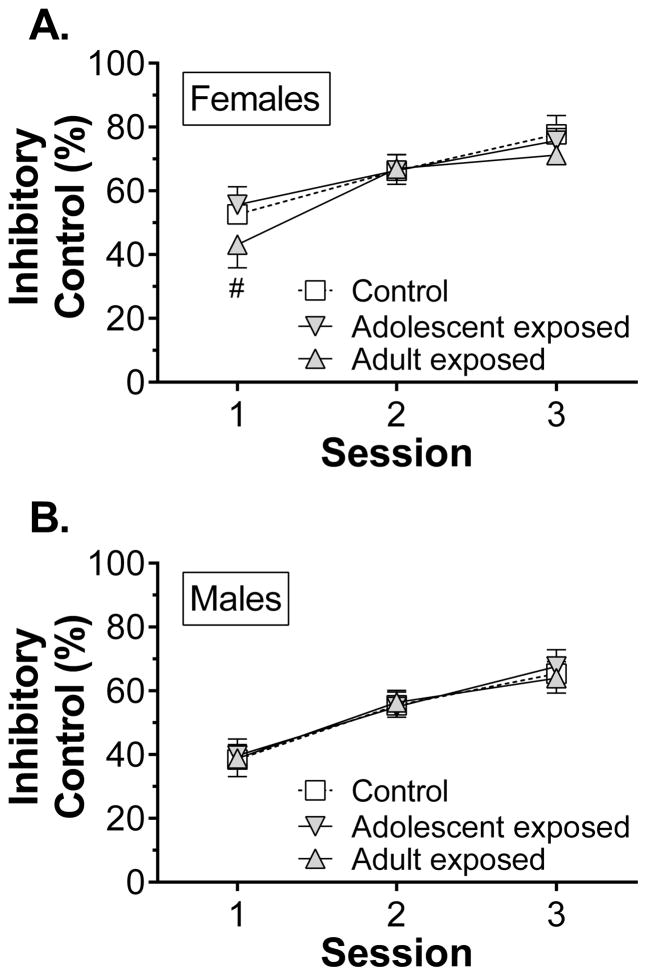
Inhibitory control during the first three sessions was influenced by interactions between sex, treatment, and delay, as discussed previously. Post hoc analysis of this interaction revealed that the effect of treatment was delay-dependent in both females (Fig. 7A) and males (Fig 7B). During the 0.5 and 1-s delays, adult-exposed females had poor inhibitory control relative to both adolescent-exposed and control rats, but they no longer differed from controls at the 2-s delay. Adolescent-exposed males, however, were only impaired relative to controls during the 2-s delay. Planned three-way ANOVA within each sex were used to explore these interactions further. In females there were main effects of treatment (F2,37 = 8.00, p = 0.001), session (F2,74 = 73.1, p < 0.001), and delay (F2,74 = 126.7, p < 0.001). Post hoc tests revealed that adult-exposed females had less inhibitory control than the adolescent-exposed and control females (Fig. 7C). In males there were main effects of session (F2,70 = 116.1, p < 0.001) and delay (F2,70 = 66.9, p < 0.001), as well as a treatment x session interaction (F4,70 = 2.96, p = 0.026). Post hoc tests revealed that adolescent-exposed males had poorer inhibitory control than control males during the 3rd session (Fig. 7D).
Figure 7.
The effect of amphetamine exposure on performance during the first three response inhibition training sessions in experiment 2 (n = 13–14/group). Panels A and B show the effect of treatment on inhibitory control for each of the premature delay durations, collapsed across session, for females and males, respectively. Panels C and D show the effect of treatment on inhibitory control for the first three sessions, collapsed across delay, in females and males, respectively. @p < 0.05, @@p < 0.01, @@@p < 0.001 vs adult-exposed females; #p < 0.05 vs adolescent-exposed males
3.2.2. Effects of intra-mPFC infusion of MK-801on response inhibition
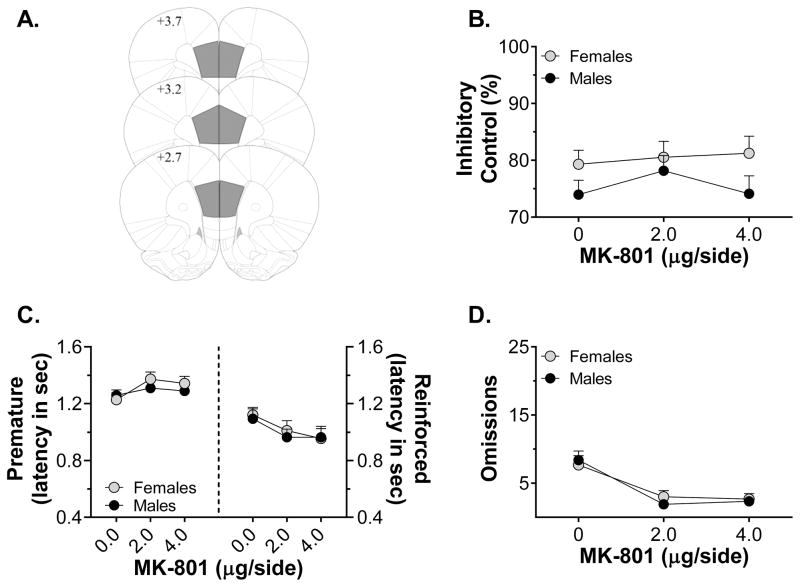
Infusion of MK-801 into the prelimbic mPFC (Fig. 8A) had no statistically significant effects on inhibitory control (Fig. 8B), but latency to make a premature response was increased (Fig. 8C). Three-way ANOVA of premature response latency revealed a main effect of dose (F2,126 = 3.55, p = 0.032), but no main effects of treatment or sex and no interactions. Latency to make a reinforced response tended to decrease across infusion dose, but three-way ANOVA revealed the main effect of dose was just below the statistical significance level (F2,122 = 3.55, p = 0.052) and there were no other significant main effects or interactions. As shown in Fig. 6D, MK-801 significantly reduced omissions. Three-way ANOVA revealed a main effect of dose (F2,126 = 19.5, p < 0.001), but no other main effects or interactions.
Figure 8.
Sex differences in response to MK-801 infusion in the mPFC, collapsed across treatment group (n = 34/group). Panel A shows the schematic representation of sites for local infusion in the mPFC. Shaded boxes indicate the area within which all included placements terminated, numbers (in mm) indicate the coronal section position relative to Bregma (sections adapted from Paxinos and Watson, 2007). Panel B shows inhibitory control following MK-801 infusion. Panel C shows latency to lever press during the premature (left) and response (right) phases. Panel D shows the number of trials omitted in each session.
4. Discussion
In the current study, we used a response inhibition task with relatively low demands on attentional resources to investigate the potentially interacting influences of sex and AMPH exposure on impulsive action. We found that females had better inhibitory control compared to males. Moreover, females were the only sex to show a consistent effect of AMPH pre-exposure on impulsivity, with rats exposed during young adulthood exhibiting reduced inhibitory control compared to rats exposed during adolescence and saline-treated controls. The effect of AMPH pre-exposure in males was limited to the second experiment, where rats exposed during adolescence, but not adulthood, displayed reduced inhibitory control relative to saline-treated controls. We also investigated whether the response to drug challenge would be affected by sex or previous AMPH exposure. In the first experiment we found that challenge injections of MK-801 or AMPH had a greater effect on task performance in females, compared to males, but the response to these challenges was unaffected by previous AMPH exposure. In addition, all AMPH-exposed rats exhibited sensitization to the locomotor-stimulating effects of acute AMPH. In the second experiment we found that intra-mPFC injections of MK-801 did not increase impulsivity and had no interacting effect with sex or AMPH exposure, but they did alter response latency and omission rate. Overall, our results suggest that sex mediates both basal (i.e., trait) and drug-induced impulsivity, as well as the response to systemic drug challenge.
4.1. Response inhibition training
The finding that females are less impulsive than males is consistent with two other studies of sex differences in impulsive action that used tasks requiring rats to attend to numerous stimuli simultaneously [21,22]. We have previously demonstrated that females exhibit faster acquisition of conditioning than males [35], and so it is possible that females may have learned to inhibit responses more rapidly than males. However, this is unlikely to explain the present results because females were less impulsive than males throughout all training and testing sessions in the first experiment. This is consistent with another recent study on sex differences in impulse control, which demonstrated that females had better inhibitory control after up to 70 sessions of training [22]. It is noteworthy, however, that that sex differences were no longer evident by the criterion session in experiment 2. Males required more sessions to reach criterion and the additional training sessions they received may have diminished the effect of sex.
Females not only make fewer premature responses, but those that they do make are delayed relative to males. One explanation for this finding is that females may have exerted more control over pre-potent responses, even when they ultimately failed to stop them. However, it is also possible that the sex difference in premature response latency was influenced by sex differences in attention. Indeed, studies demonstrating low levels of impulsive action in females have also found that females have poorer visual attention and are less able to sustain attention (i.e., vigilance) during intertrial intervals [21,22]. Though the simple response inhibition task used in this study had low attentional demands, we were able to assess visual attention through the latency to make a reinforced response after the signaled end of the premature phase; females responded at least as rapidly as males, perhaps even more rapidly, following the illumination of the cue light. Not only do females seem to have similar attention during performance of the task, but it is also unlikely that physical differences (e.g., size, speed) contributed to sex differences in premature response rate and latency.
Though sex differences in attention did not seem to inordinately contribute to our results, this does not rule out the possibility for an influencing role of vigilance [36]. When we analyzed latency during no-delay sessions, we found that females respond more slowly following the 10-s intertrial interval, even in sessions where there was no consequence for failing to inhibit responding. Combined with the slower premature response rate, this suggests that females may have poorer vigilance during the intertrial interval. Therefore, the sex difference in inhibitory control we observed may represent impaired vigilance, enhanced inhibitory control or some combination of the two. Previous work has demonstrated that these processes are connected and may influence each other [22,36]. As rats learn to inhibit premature responding in our study, they also learn that continued vigilance for the extension of the levers is no longer advantageous since the cue light becomes the relevant stimulus, instead of lever extension. Latency to respond following lever extension (i.e., during the premature phase) increases for all rats across sessions as a result of this increase in inhibitory control and decrease in vigilance. Though vigilance cannot be separated from inhibitory control in the current study, others have suggested that both factors contribute to sex differences in performance on the 5-CSRT task [22] and we suspect that this is the case in our study as well.
Though the current study adds to growing evidence for sex differences in impulsivity and vigilance, there are conflicting reports that should be considered. In one study, female rats responded more than males during signaled non-availability periods on a Go/No-Go task, which the study authors interpreted as impaired inhibitory control [37]. In contrast to other studies of impulsive action, pre-potent responses in this Go/No-Go task are without consequence, and therefore may measure differences in anticipatory or extinction responding rather than impulsive action. In another study, rats trained in a two-choice serial reaction time (2-CSRT) task exhibited inconsistent sex differences in impulsive action [38]. It is noteworthy, however, that females are less impulsive than males when tested with the more complex, five-choice version of this task, the 5-CSRT task [22]. The reason for the discrepancy between these similar tasks is unclear and warrants further investigation. Finally, it is possible that sex differences in impulsivity are subtype-dependent as female rats exhibit greater levels of impulsive choice [20]. In contrast, human females seem to have lower levels of impulsive choice [19,39]. More human and animal studies are needed to clarify how sex affects impulsivity as differences in impulsivity may lead to meaningful changes in disease vulnerability or even in the effects of drugs of abuse.
We found that the effects of AMPH exposure on impulsive action were dependent on sex and age of exposure. Specifically, females exposed to AMPH in adulthood exhibited increased impulsivity compared to saline-treated controls. Though we found that adult-exposed females were more impulsive in both experiments, the effect of AMPH exposure on impulse control was more pronounced in the second experiment. For males, we found no effect of AMPH exposure in the first experiment, while there was a modest effect of adolescent-exposure in the second. The reasons for this are not clear, but rats in the second experiment underwent surgery for bilateral implantation of intracerebral cannulae and this experience could be considered a stressful event. Stress can cause cross-sensitization to the effects of psychomotor stimulants [40,41]. Therefore, the stress induced by surgery may have interacted with the effects of prior AMPH exposure to cause even greater drug-induced deficits in impulse control in the second experiment.
Though AMPH exposure produced increases in impulsivity in both males and females, the effect was more robust and consistent in females. Our finding that females were more impacted by the long-term effect of AMPH on impulsive action is consistent with a recent study in humans, which found that psychostimulant abuse is associated with greater increases in motor impulsivity in females, relative to males [42]. Increased sensitivity to the effects of AMPH on the mPFC could explain the observed sex difference in AMPH-induced impulsivity [29,43]. For example, AMPH sensitization leads to reduced PFC thickness in females, but not in males [44]. Though we found that females were more sensitive to the effects of AMPH on impulse control, we failed to reveal an effect of sex on locomotor sensitization. Although this seems inconsistent with other published results [29,31], these studies assessed sensitization on the last day of AMPH exposure. Here, we used an extended withdrawal period (two to four months, depending on age-of-exposure). Given that sex differences disappear when sensitization is examined following just 16 days of withdrawal [45], the longer withdrawal period in the current study may explain the similar levels of sensitization in males and females.
Though adult AMPH exposure produced more robust effects in females, only males demonstrated persistent impairments following adolescent AMPH exposure. The finding that adolescent exposure, but not adult exposure, increased impulsivity in males is consistent with our previous studies in males, where we have demonstrated that adolescent exposure to AMPH has a greater effect on working memory and behavioral flexibility compared to adult exposure [24,25]. Adolescents may be more vulnerable to the effects of drugs because adolescence is an important period in cortical development [46]. However, we were unable to detect any persistent effect of adolescent AMPH-exposure on impulsivity in females even though sensitization to the locomotor-stimulating effects of AMPH was still evident nearly four months after the last AMPH treatment in adolescence. Sex differences in cortical development may explain the lack of effect in females. Females undergo overproduction and pruning of synapses earlier in adolescence than males; therefore males and females may have been in different stages of cortical development during the final adolescent AMPH treatments [47].
It is also possible that adolescent-exposed females may have recovered from the effects of repeated AMPH exposure on impulsivity by the time training in the task commenced. Adult-exposed rats began training after a withdrawal period of 17–27 days, whereas adolescent-exposed rats began after an 80–90 day withdrawal period. In our previous study, the effect of adolescent AMPH-exposure on working memory was diminished when training began after an 80-day, relative to a 30-day, withdrawal period [24]. In addition, others have shown that deficits in memory caused by adolescent cocaine exposure are no longer apparent following a 105 day withdrawal period [48]. Also noteworthy are results showing that adolescent rats exposed to cocaine do not exhibit long term changes in nucleus accumbens glutamate following a 14-day withdrawal whereas adult-exposed rats do exhibit these changes [49–51].
4.2. Effects of systemic drug challenges
Although adult-exposed females exhibited AMPH-induced increases in impulsive action, AMPH exposure did not influence the effects of MK-801 and AMPH challenge on impulsive action. Systemic challenge with the NMDA antagonist MK-801, which has previously been shown to increase impulsive action at similar doses [34], decreased inhibitory control but there was no difference between AMPH-exposed and control animals. We did, however, find that MK-801 was significantly more effective at disrupting task performance (response latency and omissions) in females compared to males. Previously, females were reported to exhibit more locomotor behavior and neurotoxicity following high doses (0.3 and 5.0 mg/kg, respectively) of MK-801 [52,53]. One potential interpretation of the heightened sensitivity of females to the effects of MK-801 that we and others have observed is that there may be sex differences in glutamatergic transmission. There is currently little direct evidence for such a hypothesis, but others have suggested that sex differences in glutamatergic signaling could help explain the fact that female rats tend to exhibit enhanced performance in cognitive and instrumental conditioning tasks relative to males [54,55]. Of course, sex differences in sensitivity to MK-801 likely also would involve other neurochemical systems as this drug leads to increased metabolism and turnover of dopamine and serotonin in many areas, including the PFC [56]. These systems have all been shown to be involved in impulse control [57,58]. For example, poor inhibitory control is associated with higher levels of dopamine D1 receptor mRNA expression in the nucleus accumbens [59] and male rats have greater levels of nucleus accumbens D1 receptor expression [60].
The sex differences we observed in response to AMPH challenge followed the same pattern that we observed with challenge injections of MK-801: females exhibited heightened sensitivity to the effects of AMPH challenge on reinforced response latency and omissions. This provides additional support for the possibility that sensitivity to the disruptive effects of MK-801 may be mediated by sex differences in dopamine signaling. As with MK-801, we found a dose-dependent decrease in inhibitory control following AMPH challenge, but we found no effect of previous AMPH exposure on the response to AMPH challenge. In contrast, all AMPH-exposed rats exhibited robust sensitization to the locomotor stimulating effects of AMPH, regardless of sex or age-of-exposure. This suggests that the changes underlying the development of locomotor sensitization do not extend to areas involved in impulse control. In line with this, AMPH sensitization produces changes in monoamine transmission in numerous regions, but not within the mPFC [45,61]. Given that the mPFC plays an important role in the control of impulsive responses, it would seem that locomotor sensitization to AMPH does not necessarily predict changes in the effect of AMPH on mPFC-related tasks.
4.3. Effects of intra-mPFC infusion of MK-801on response inhibition
Previous studies have suggested that acquisition and performance of a response inhibition task similar to the one used here is associated with changes in the expression of AMPA and NMDA receptors in the mPFC [23]. This led us to hypothesize that intra-mPFC infusions of an NMDA antagonist would disrupt task performance. However, we found that intra-mPFC infusions of MK-801 disrupted response latency and omissions while having no significant effect on inhibitory control. Thus, we observed an effect of systemic, but not intra-mPFC, MK-801 on inhibitory control. There are a number of factors that may be responsible for this discrepancy. First, systemic MK-801 affects glutamate, dopamine and serotonin in multiple brain regions [56], so it is possible that the effects of systemic MK-801on impulsive action may not have occurred in the mPFC. Another possibility is that the doses of MK-801 we infused into the mPFC may not have been high enough to produce an effect. This is somewhat unlikely, however, since similar doses of MK-801 infused into the mPFC impair performance on other tasks designed to test behavioral flexibility [62,63] and working memory [64]. A third possibility is related to the target location for our infusions, the prelimbic region of the mPFC. This region was targeted because plasticity associated with learning the response inhibition task seems to occur in the prelimbic, and not the infralimbic, mPFC [23]. However, intra-mPFC infusion of another NMDA antagonist, R-CPP, increased impulsivity on the 5-CSRTT when the infusion was aimed at the infralimbic, but not the prelimbic, mPFC [65]. Furthermore, mPFC lesions increase impulsivity on the 5-CSRTT when they occur in the infralimbic, but not the prelimbic, mPFC [66]. Because we did not find an effect of intra-mPFC MK-801 in any of our rats, it is unclear whether AMPH exposure affected glutamate transmission in this region.
4.4. Conclusion
We found that females had better inhibitory control, but poorer vigilance, relative to males, which is an effect we replicated across two different experiments. When taken with previous results [21,22], these findings suggest that the female advantage in inhibitory control may be independent of sex differences in attention, but is not necessarily independent of sex differences in vigilance. Though females were less impulsive in general, their behavior was also more affected by drug exposure and challenge. Females exhibited drug-induced impulsivity following exposure to a sensitizing regimen of AMPH during adulthood and were more sensitive to the effects of MK-801 and AMPH challenge. Males, in contrast, only exhibited a modest degree of drug-induced impulsivity following adolescent exposure to AMPH. The heightened sensitivity of females to drug effects may represent a sex difference in sensitivity to these drugs. Though we found that all AMPH-exposed rats exhibited locomotor sensitization to the effects of the drug, this sensitization was not associated with any change in the effects of AMPH or MK-801 challenge. Because we were unable to reduce impulse control with intra-mPFC infusion of MK-801, these results cannot speak to the effect of AMPH exposure on mPFC glutamate transmission. In summary, we found that sex mediates impulsivity and the response to drug challenge, while sex and age-of-exposure interact to mediate the effects of AMPH exposure on impulse control. Though females may be less susceptible to developing diseases like ADHD and addiction because of their enhanced inhibitory control, they may also be more vulnerable to the harmful long-term effects of drug abuse.
We studied impulsive action and vigilance in adult male and female rats
Rats were pre-exposed to saline or amphetamine in adolescence or young adulthood
Females were less impulsive than males and have poorer vigilance
Impulsivity was increased in adult-exposed females and adolescent-exposed males
Females were more affected by AMPH and MK-801 challenge, relative to males
Acknowledgments
This study was supported by a grant from the National Institute on Drug Abuse (R01 DA029815). We thank John Weaver, Sapan Shah, Rakesh Marreddy and Olubankole Aladesuyi Arogundade for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 3.Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: implications for alcoholism. Alcohol Clin Exp Res. 2010;34:1319–33. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2009;33:690–8. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jentsch J, Pennington Z. Reward, interrupted: inhibitory control and its relevance to addictions. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Bari A, Robbins TW. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog Neurobiol. 2013 doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 9.Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–73. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- 10.Colzato LS, van den Wildenberg WPM, Hommel B. Impaired inhibitory control in recreational cocaine users. PLoS One. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–7. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Trent S, Davies W. The influence of sex-linked genetic mechanisms on attention and impulsivity. Biol Psychol. 2012;89:1–13. doi: 10.1016/j.biopsycho.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon J. A Meta-Analytic Review of Gender Differences in ADHD. J Atten Disord. 2002;5:143–54. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- 15.Holden C. Sex and the suffering brain. Science. 2005;308:1574–7. doi: 10.1126/science.308.5728.1574. 80- [DOI] [PubMed] [Google Scholar]
- 16.Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28:17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JM, Link BG. Gender and the expression of schizophrenia. J Psychiatr Res. 1988;22:141–55. doi: 10.1016/0022-3956(88)90078-7. [DOI] [PubMed] [Google Scholar]
- 18.Miller LS, Burns SA. Gender differences in schizotypic features in a large sample of young adults. J Nerv Ment Dis. 1995;183:657–61. doi: 10.1097/00005053-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Dev Neuropsychol. 2008;33:179–96. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- 20.Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–37. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jentsch JD, Taylor JR. Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav Neurosci. 2003;117:76–83. doi: 10.1037//0735-7044.117.1.76. [DOI] [PubMed] [Google Scholar]
- 22.Bayless DW, Darling JS, Stout WJ, Daniel JM. Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. Behav Brain Res. 2012;235:48–54. doi: 10.1016/j.bbr.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Hayton SJ, Lovett-Barron M, Dumont EC, Olmstead MC. Target-Specific Encoding of Response Inhibition: Increased Contribution of AMPA to NMDA Receptors at Excitatory Synapses in the Prefrontal Cortex. J Neurosci. 2010;30:11493–500. doi: 10.1523/JNEUROSCI.1550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hankosky E, Kofsky N, Gulley J. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–25. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hankosky E, Gulley J. Performance on an impulse control task is altered in adult rats exposed to amphetamine during adolescence. Dev Psychobiol. 2012 doi: 10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker JB, Robinson TE, Lorenz KA. Sex difference and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- 28.Castner SA, Xiao L, Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–34. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- 29.Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 30.Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 31.Mathews IZ, McCormick CM. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behav Pharmacol. 2007;18:641–50. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- 32.Lu W, Wolf ME. Repeated amphetamine administration alters AMPA receptor subunit expression in rat nucleus accumbens and medial prefrontal cortex. Synapse. 1999;32:119–31. doi: 10.1002/(SICI)1098-2396(199905)32:2<119::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Lu W, Monteggia LM, Wolf ME. Withdrawal from repeated amphetamine administration reduces NMDAR1 expression in the rat substantia nigra, nucleus accumbens and medial prefrontal cortex. Eur J Neurosci. 1999;11:3167–77. doi: 10.1046/j.1460-9568.1999.00736.x. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–77. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Hammerslag LR, Gulley JM. Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol. 2013 doi: 10.1002/dev.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 37.Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–29. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- 38.Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: The role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 39.Kirby KN, Maraković NN. Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychon Bull Rev. 1996;3:100–4. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- 40.Nikulina EM, Covington HE, Ganschow L, Hammer RP, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–65. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 41.Doremus-Fitzwater TL, Spear LP. Age-related differences in amphetamine sensitization: effects of prior drug or stress history on stimulant sensitization in juvenile and adult rats. Pharmacol Biochem Behav. 2010;96:198–205. doi: 10.1016/j.pbb.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry RI, Krmpotich T, Thompson LL, Mikulich-Gilbertson SK, Banich MT, Tanabe J. Sex modulates approach systems and impulsivity in substance dependence. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Muhammad A, Hossain S, Pellis SM, Kolb B. Tactile stimulation during development attenuates amphetamine sensitization and structurally reorganizes prefrontal cortex and striatum in a sex-dependent manner. Behav Neurosci. 2011;125:161–74. doi: 10.1037/a0022628. [DOI] [PubMed] [Google Scholar]
- 45.Bisagno V, Ferguson D, Luine VN. Chronic d-amphetamine induces sexually dimorphic effects on locomotion, recognition memory, and brain monoamines. Pharmacol Biochem Behav. 2003;74:859–67. doi: 10.1016/s0091-3057(03)00017-0. [DOI] [PubMed] [Google Scholar]
- 46.Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicol Teratol. 2004;26:651–61. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Marin MT, Cruz FC, Planeta CS. Cocaine-induced behavioral sensitization in adolescent rats endures until adulthood: lack of association with GluR1 and NR1 glutamate receptor subunits and tyrosine hydroxylase. Pharmacol Biochem Behav. 2008;91:109–14. doi: 10.1016/j.pbb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–13. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 51.Scheggi S, Mangiavacchi S, Masi F, Gambarana C, Tagliamonte A, De Montis MG. Dizocilpine infusion has a different effect in the development of morphine and cocaine sensitization: behavioral and neurochemical aspects. Neuroscience. 2002;109:267–74. doi: 10.1016/s0306-4522(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 52.Devaud LL, Bartoo G, Malthankar G. Altered responses to dizocilpine maleate administration in ethanol-withdrawn male and female rats. Alcohol. 2002;28:83–93. doi: 10.1016/s0741-8329(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 53.Hur GH, Son WC, Shin S, Kang JK, Kim YB. Sex differences in dizocilpine (MK-801) neurotoxicity in rats. Environ Toxicol Pharmacol. 1999;7:143–6. doi: 10.1016/s1382-6689(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 54.Sutcliffe JS. Female rats are smarter than males: influence of test, oestrogen receptor subtypes and glutamate. In: Neill JC, Kulkarni J, editors. Biol Basis Sex Differ Psychopharmacol. Berlin: Springer-Verlag Berlin Heidelberg; 2011. pp. 37–56. [DOI] [PubMed] [Google Scholar]
- 55.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–38. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Löscher W, Annies R, Hönack D. The N-methyl-D-aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats. Neurosci Lett. 1991;128:191–4. doi: 10.1016/0304-3940(91)90258-u. [DOI] [PubMed] [Google Scholar]
- 57.Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–49. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- 59.Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37:1779–88. doi: 10.1111/ejn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 61.Scholl JL, Feng N, Watt MJ, Renner KJ, Forster GL. Individual differences in amphetamine sensitization, behavior and central monoamines. Physiol Behav. 2009;96:493–504. doi: 10.1016/j.physbeh.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005;119:420–8. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- 63.Watson DJ, Stanton ME. Medial prefrontal administration of MK-801 impairs T-maze discrimination reversal learning in weanling rats. Behav Brain Res. 2009;205:57–66. doi: 10.1016/j.bbr.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jablonski SA, Watson DJ, Stanton ME. Role of medial prefrontal NMDA receptors in spatial delayed alternation in 19-, 26-, and 33-day-old rats. Dev Psychobiol. 2010;52:583–91. doi: 10.1002/dev.20465. [DOI] [PubMed] [Google Scholar]
- 65.Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- 66.Pattij T, Vanderschuren LJMJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–9. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]