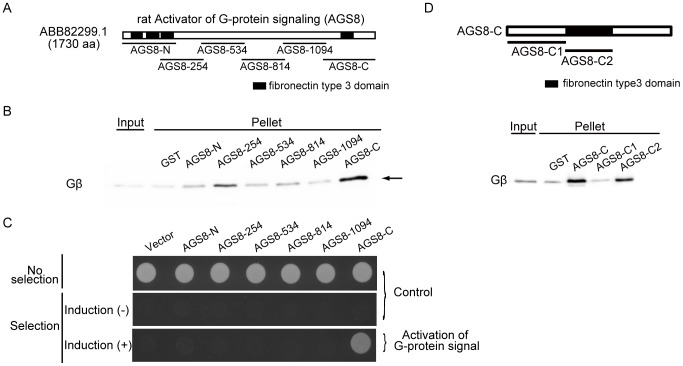
Figure 1. Determination of Gβγ interacting domain of the AGS8.
(A) Schematic diagram of rat AGS8 and the AGS8 domains synthesized as GST-fusion proteins. Each GST protein fused with the following segment of rat AGS8 (ABB82299) respectively. AGS8-N: M1- P370, AGS8-254: A254– R553, AGS8-534: S534– S833, AGS8-814: S814– R1113, AGS8-1094: H1094– D3280, AGS8-C: A1359– W1730. (B) GST-pulldown assay of AGS8 domains with Gβ1γ2. AGS8 domains synthesized as GST-fusion proteins (300 nM) were incubated with recombinant human Gβ1γ2 (30 nM) in a total volume of 300 µl at 4°C. Proteins were then adsorbed to a glutathione matrix and retained G-protein subunits identified by immunoblotting following gel electrophoresis. The representative of 5 independent experiments with similar results. (C) Bioactivity of AGS8 domains on G-protein activation in cell. The yeast strain expressing human Gαs was transformed with AGS8 domains described in (A) into the pYES2-containing GAL1 promoter. The yeast strain was modified to grow without histidine on activation of G-protein. Induction(+): induction of translation of AGS8 domains by galactose. The representative of 4 independent experiments with similar results. (D) GST-pull down assay of AGS8-C segments with recombinant Gβγ. (upper panel) Schematic diagram of AGS8-C and the segments synthesized as GST-fusion proteins. Each GST protein fused with the following segment of rat AGS8 (ABB82299) respectively. AGS8-C1: A1359– H1493, AGS8-C2: A1494– T1585. (lower panel) GST-pulldown assay of AGS8 segments with Gβ1γ2. AGS8 domains synthesized as GST-fusion proteins (300 nM) were incubated with recombinant human Gβ1γ2 (30 nM) in a total volume of 300 µl at 4°C. Proteins were then adsorbed to a glutathione matrix and retained G-protein subunits identified by immunoblotting following gel electrophoresis. The representative of 4 independent experiments with similar results.