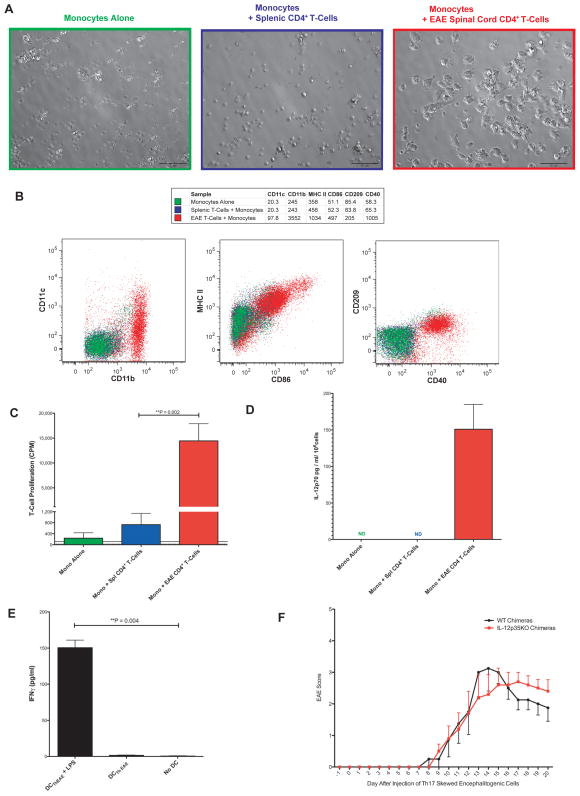
Figure 7. Infiltrating EAE Th-cells Induce the Formation of Th1 Skewing Mo-DC.
A) CD4+ T-cells from EAE mice spinal cords or a healthy wild-type spleen cells were cultured 1:10 with monocytes alone or monocytes in the presence of 10ng/ml IL-2 for 4 days and then imaged with bright field microscopy. The scale bar represents 50 microns. The results are representative of 4 independent experiments. B) Cells from A) were gated on CD4−, CD11b+, DAPI− and analyzed by flow cytometry. MFI for each marker is shown in the table above. Results are representative of 3 independent experiments. C) Cells from A) were harvested, pulsed with MHC II-restricted OVA peptide and then cultured in triplicate at a ratio of 1 APC:33 naïve T-cells from OT-II RAG KO mice for 3 days and then pulsed with H3 thymidine for 20 hours. The dotted horizontal line represents the mean proliferation of OT-II T-cells in the absence of APC. The solid horizontal line represents the mean proliferation of the DCThEAE without OT-II T-cells. Cultures were performed in triplicate. D) Cells prepared as in A) were stimulated overnight with 1μg/ml of LPS. The cell-free supernatant was assayed in duplicate by ELISA for IL-12p70. Data shown are representative of 2 independent experiments. ND = Not Detected. E) CD11b+ DCThEAE selected via MACS were pulsed with or without 1μg/ml LPS and 2.5μg/ml OVA peptide for 90 minutes, washed and then cultured at a ratio of 1 APC: 5 naïve T-cells from OT-II RAG KO mice for 5 days. Supernatant was collected and assayed via ELISA for IFNγ. Done in triplicate. F) BM chimeric mice were generated using wild-type or IL-12p35 KO BM. 10 weeks later, the mice were injected with Th17 skewed encephalitogenic cells to induce EAE, and mice were monitored for signs of disease on a daily basis. N = 4–5 mice per group. Data are presented as means ± SEM, with significance determined by a two-tailed Mann-Whitney test. Day 17 p = 0.27, Day 18 p = 0.36, Day 19 p = 0.37, Day 20 p = 0.45