Abstract
The aim of the study was to investigate the differences in anticipatory (APAs) postural adjustments between young and older adults and its effect on subsequent control of posture. Ten healthy older adults and thirteen healthy young adults were exposed to predictable external perturbations using the pendulum-impact paradigm. EMG activity of the trunk and leg muscles, the center of pressure (COP), and center of mass (COM) displacements in the anterior-posterior (AP) direction were recorded and analyzed during the anticipatory and compensatory (CPAs) phases of postural control. The effect of aging was seen as delayed anticipatory muscle activity and larger compensatory muscle responses in older adults as compared to young adults. Moreover, in spite of such larger reactive responses, older adults were still more unstable, exhibiting larger COP and COM peak displacements after the perturbation than young adults when exposed to similar postural disturbances. Nonetheless, while APAs are impaired in older adults, the ability to recruit muscles anticipatorily is largely preserved, however, due to their smaller magnitudes and delayed onsets, it is likely that their effectiveness in reducing the magnitude of CPAs is smaller. The outcome of the study lends support towards investigating the ways of improving anticipatory postural control in people with balance impairments due to aging or neurological disorders.
Keywords: Posture, anticipatory, compensatory, aging
Introduction
Bipedal stance in humans has stringent stability demands and is usually associated with maintenance of vertical orientation. The ability to maintain the body’s center of mass (COM) over the base of support during quiet stance, movement, and in response to balance threats is one of the main goals of postural control (Hess et al. 2005). Efficient balance control is also fundamental to the successful performance of all activities of daily living (ADL) and in essence it shadows human mobility. This relationship between posture, balance and intentional movements is particularly important in the elderly population where a generalized decrease in balance control is accompanied by an increased frequency of movement related falls (Rogers et al. 1992). Moreover, the relationship between balance control and independent mobility becomes vital in older adults where poor postural control is associated with significant mobility losses (Frank and Patla 2003), physical inactivity and an increase in the fear of falling (Skelton and Beyer 2003).
The central nervous system (CNS) uses anticipatory and compensatory postural strategies to maintain and restore balance when perturbed. Anticipatory postural adjustments (APAs) reflect a feedforward control mechanism wherein changes are seen in the background activity of muscles prior to an upcoming postural perturbation. APAs are based on previous experiences or anticipation and help in minimizing potential disturbances to balance due to an expected external perturbation or a forthcoming self-initiated movement (Belen'kii et al. 1967; Bouisset and Zattara 1987; Massion 1992; Aruin and Latash 1995). Compensatory postural adjustments (CPAs) on the other hand are a feedback based control mechanism wherein changes in muscle activity are seen following a perturbation. They deal with the actual effects of a postural disturbance and help in restoring balance after a perturbation has occurred (Nashner and Cordo 1981) (Horak and Nashner 1986). CPAs are triggered by sensory feedback signals in response to the perturbation (Park et al. 2004; Alexandrov et al. 2005). Postural control in humans is based on the effective use of anticipatory and compensatory postural mechanisms. In the case of an unexpected perturbation to posture, CPAs are the only mechanism used by the CNS to restore balance. On the other hand, when the perturbation is predictable, APAs act as the first line of defense preparing the body for the upcoming disturbance and are thereafter followed by CPAs that help in completing the process of balance restoration. Nonetheless, the utilization of APAs significantly reduces the need for large CPAs and results in greater postural stability as demonstrated by significantly small displacements of the body’s COM and center of pressure (COP) following a perturbation in healthy young adults (Santos et al. 2010b; Santos et al. 2010a). These findings highlight the importance of APAs in control of posture, and point out the existence of a relationship between anticipatory and compensatory components of postural control.
Prior studies of APAs in the elderly were mainly based on using self-initiated movements. Thus, it was demonstrated that APA activity associated with self generated body perturbations is significantly delayed in the healthy elderly, with postural muscles being recruited closer to the activation of prime mover muscles (Man'kovskii et al. 1980; Inglin and Woollacott 1988; Rogers et al. 1992; Woollacott and Manchester 1993) or after prime mover activation (Woollacott and Manchester 1993). A parallel worsening of the quality of performance of the motor task was also observed with an increase in the frequency of loss of balance (Man'kovskii et al. 1980). Moreover, the classic distal to proximal muscle activation pattern was found to be disrupted in the elderly. A study on push and pull arm movements reported that contrary to the classical activation pattern seen in young subjects, older subjects exhibited patterns where either the distal and proximal muscles were activated on opposite body aspects (for push movements), or patterns where only distal muscles were activated (for pull movements) (Inglin and Woollacott 1988). It was also reported that while performing an unilateral arm raising task under the self-paced condition at maximal velocity, elderly subjects used a “hip strategy” like cluster of activation of muscles as compared to the young who used activation of muscles that resembled an “ankle strategy”. In addition, when the same task was performed under the externally triggered condition at maximal velocity, the strategy involved an increased activation of certain thigh muscles, rather than a sequence modification. It was suggested that the elderly adopted different muscle strategies in order to perform the same movement (as the young) in the presence of reduced stability induced by delayed postural preparation (Bleuse et al. 2006). Aging, however, does not seem to affect anticipatory recruitment of postural muscles (Rogers et al. 1992; Garland et al. 1997; Bleuse et al. 2006). Elderly subjects have been found to recruit their lower limb and trunk muscles prior to a focal movement in 96–100% of trials which is as frequent as the young subjects (Rogers et al. 1992). Thus, it seems that deficits in postural control with aging do not appear to be due to an absence of APAs, which have otherwise been found to be sometimes absent in neurological conditions of the aged such as Parkinson’s disease (Bazalgette et al. 1987).
Age-related changes in anticipatory postural control have also been reflected in terms of biomechanical parameters. An investigation of APAs in the elderly, focused on the center of pressure (COP) excursion during performance of fast arm movements, found no statistical difference between the elderly and young groups (Garland et al. 1997). On the other hand, a study involving an unilateral arm raising task in the elderly, demonstrated that at maximal velocity, vertical torque (Tz) was delayed in all conditions (self-paced with and without load and externally triggered), whereas COP onset was delayed only in the self-paced condition without load. At low velocity, however, elderly subjects did not show any impairment in stability (Bleuse et al. 2006).
In conjunction with delayed APA activity of postural muscles, large significant increases in the onset latencies of the voluntary (prime mover) muscles were also seen in the elderly during performance of rapid push and pull arm movements in comparison to young adults (Inglin and Woollacott 1988). Experiments using the rapid arm raising paradigm, demonstrated prolonged movement times and reduced peak arm accelerations in the elderly as compared to the younger group for both, self-paced (Rogers et al. 1992; Garland et al. 1997) and reaction time movements (Rogers et al. 1992). However, contrasting findings have been reported during performance of arm raising movements at different velocities, with results showing no statistical differences between the elderly and the younger groups. That is, for a given velocity both the groups showed similar movement performance (Bleuse et al. 2006).
Consequently, the understanding of APAs in the elderly is obscured by two main contrasting issues: whether lack of adequate postural activity affects movement performance or whether impaired anticipatory postural activity in the elderly is dependent on the movement quality? This contradiction could be attributed to the fact that almost all of the previous investigations involved self generated body perturbations (such as push and pull tasks, arm raising tasks under self-paced or reaction time conditions), where characteristics of the voluntary movement may have acted as a mediating variable between aging and postural preparation. In order to have a clear understanding of how aging affects the organization of APAs, it is essential to distinguish the influence of voluntary movement performance and postural preparation. In the recent past, external perturbations were used to study anticipatory postural control. The studies involving external perturbations such as load catching (Aruin et al. 2001; Shiratori and Latash 2001) and pendulum impact paradigms (Santos and Aruin 2008; Santos et al. 2010a; Mohapatra and Aruin 2013) revealed that APAs could be seen in preparation to perturbations that were externally induced with no voluntary movement being performed by the subjects themselves. Thus as long as the perturbation is predictable (either external or internal in origin), APAs are observed with patterns that are adequate for counteracting the expected impact. However, external predictable perturbations provide a distinct advantage of delivering consistent postural disturbances that are independent of the variability inherent to the voluntary movement performance. In addition, using an external perturbation paradigm eliminates the influence occurring from the interaction between voluntary movement and postural control. Therefore, to avoid the possible interaction with a focal voluntary movement (such as during pushing or arm raising tasks) and to investigate the direct effect of aging on the anticipatory postural processes per se, using an external predictable perturbation, the magnitude of which is consistent across the subjects, will allow minimizing the confounding effects of the voluntary movement itself.
Moreover, given that the availability of robust APAs in young adults reduces the need for compensatory postural reactions and enhances postural stability following a perturbation to balance, it is pertinent to understand how aging affects the functional role of APAs in subsequent balance control of the elderly. Inability of the older adults to optimally generate postural adjustments prior to an upcoming balance threat may put additional demands on their postural control system, thereby, placing them at a greater risk for losing balance. Understanding the generation and utilization of APAs in balance control of the elderly will also create a background for investigating the role of training in improving anticipatory postural control in people with balance impairments due to aging or neurological disorders.
This study was thus focused on investigating the differences in anticipatory postural adjustments between young and older adults and its effect on subsequent control of posture using external predictable perturbations. We hypothesized that in older adults APAs will be delayed and reduced in magnitude and will be associated with the presence of large CPAs. As a result, peak COP and COM displacements following a perturbation will be greater in older than young adults. The study utilized a pendulum impact paradigm that allows inducing external predictable perturbations of same magnitudes, thereby eliminating the effect of age-related changes in voluntary movement performance on postural preparation.
Methods
Subjects
Thirteen healthy young adults (7 males and 6 females) and ten healthy older adults (6 males and 4 females) participated in the study. The mean age of the young adults group was 26.69 ± 3.72 years; mean body mass 68.10 ± 13.61 kg, and mean height 1.74 ± 0.09 m. The mean age of the older adults group was 69.9 ± 4.04 years; mean body mass 76.42 ± 17.39 kg, and mean height 1.70 ± 0.13 m. The young adults were recruited from the university student population while the older adults were recruited from the community and all were screened for eligibility to participate. All participants were able to understand and follow instructions, were independent in their mobility, had normal or corrected to normal vision, were not on any sedative medications or medications that affected their cognitive functioning, and had not undergone any surgery in the six months prior to study participation. They all signed a written informed consent approved by the University’s Institutional Review Board.
Experimental Set-up and Procedure
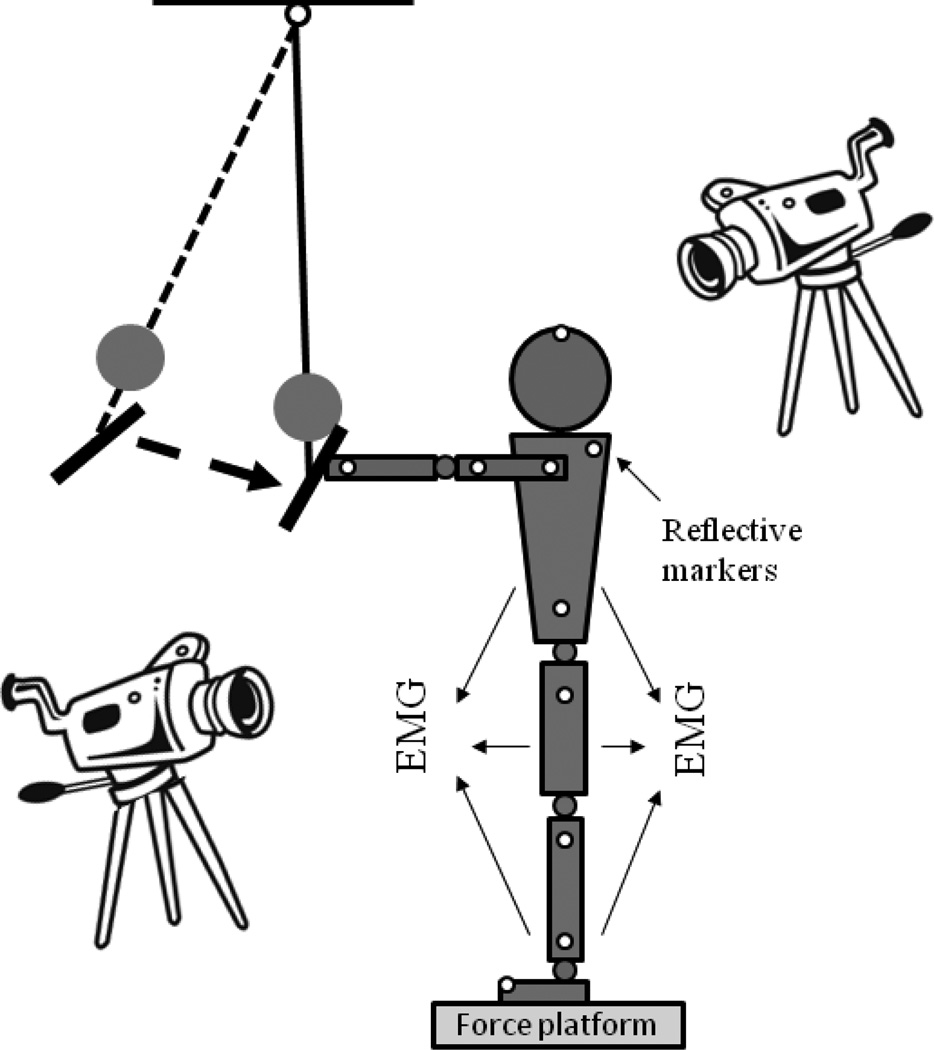
The subjects were instructed to maintain upright stance while standing barefoot on the force platform with their feet shoulder width apart. The pendulum impact paradigm was used to perturb the subjects. A load (mass, m = 3% of the subjects’ body weight) was attached to the pendulum next to its distal end. The subjects were required to receive each pendulum impact with their hands, while their arms, wrists, and fingers were extended at the shoulder level (Fig. 1), and to maintain their balance after the perturbation. Both the groups received a series of perturbations while their eyes were open; the perturbations were thus predictable and hence elicited both, APAs and CPAs. Two to three practice trials were given to both the groups. For safety purposes, the subjects wore a harness (NeuroCom, USA) with two straps attached to the ceiling. Ten trials were performed, each five seconds in duration. All participants were allowed to have rest periods as needed.
Figure 1.
Schematic representation of the experimental setup.
Instrumentation
Electromyographic (EMG) activity of muscles was recorded from thirteen right lower limb and trunk muscles: soleus (SOL), lateral gastrocnemius (GASL), medial gastrocnemius (GASM), tibialis anterior (TA), rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), biceps femoris (BF), semitendinosis (ST), gluteus medius (GMED), external oblique (EO), rectus abdominis (RA), and erector spinae longus (ESL). Based upon recommendations reported in previous literature (Basmajian and Blumenstein 1980), disposable electrodes (Red Dot 3M) were attached to the muscle belly of each of the above muscles after cleaning the skin area with alcohol wipes. A ground electrode was attached to the anterior aspect of the leg over the tibial bone. EMG signals were collected, filtered, and amplified (10–500 Hz, gain 2000) with the EMG system (Myopac, RUN Technologies, USA). A force platform (model OR-5, AMTI, USA) was used to record the ground reaction forces and moments of forces. The signal obtained from an accelerometer (Model 208CO3, PCB Piezotronics, Inc, USA) attached to the pendulum was used to register the moment of the pendulum impact.
Three-dimensional kinematic data was collected using a six-camera VICON 612 system (Oxford Metrics). Retroreflective markers were placed over anatomical landmarks bilaterally according to the Plug-In-Gait (PIG) model (Oxford Metrics), which includes: second metatarsal head, calcaneus, lateral malleolus, lateral epicondyle of the femur, a marker on the lateral border of the leg (between the lateral malleolus and femoral epicondyle markers), anterior/posterior superior iliac spines, a marker on the lateral border of the thigh (between the femoral epicondyle and anterior superior iliac spines), second metacarpal, lateral epicondyle of the humerus, acromio-clavicular joint, and a marker on the lateral border of the arm (between the humeral epicondyle and the acromio-clavicular joint markers). Also, subjects wore head and wrists bands with 4 and 2 markers attached on them, respectively. Finally, 5 additional markers were attached over the following landmarks: 7th cervical vertebra, 10th thoracic vertebra, inferior angle of the right scapula, between the 2 sternoclavicular joints, and xiphoid process of the sternum bone.
The VICON 612 data station controlled data collection of all signals such that forces, moments of force, EMG, and accelerometer signals were acquired at 1000 Hz and kinematic data were collected at 100 Hz.
Data Processing
The data were analyzed off-line using MATLAB (MathWorks, Natick, MA) programs. Five to seven trials were used for further analysis. EMG signals were rectified and filtered with a 100 Hz low-pass, 2nd order, zero-lag Butterworth filter, while the ground reaction forces, moments, and COM data were filtered with a 40 Hz low-pass, 2nd order, zero-lag Butterworth filter. The ‘time-zero’ (T0=0, moment of pendulum impact) was calculated from the accelerometer signal as a point in time at which the signal exceeded 5% of the maximum acceleration. This value was confirmed by visual inspection by an experienced researcher. Data in the range from −600 ms (before T0) to +1000 ms (after T0) were selected for further analysis. Individual trials were aligned according to T0 and this was used as a common reference point for all the signals.
The muscle onset or muscle latency (beginning of activation/inhibition of a muscle) for each trial was detected in a time window from −250 ms to +250 ms in relation to T0 by a combination of computer algorithm and visual inspection of the trials. The latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was greater (activation) or smaller (inhibition) than the mean ± 2 SD of its baseline value, measured from −500 to −400 ms. The onset latencies for each muscle were then averaged across the trials for each subject.
Integrals of anticipatory and compensatory EMG activity were derived using average trials for each subject. Integrals of the EMG activities (IntEMGi) were calculated for 4 different epochs, each of 150 ms duration in relation to T0. The time windows for the 4 epochs were: 1) from −250 ms to −100 ms (anticipatory adjustments, APA1); 2) −100 ms to +50 ms (anticipatory adjustments, APA2) (Shiratori and Latash 2001; Santos et al. 2010a; Mohapatra et al. 2012); 3) +50 ms to 200 ms (compensatory reactions, CPA1); and 4) + 200 ms to 350 ms (late compensatory reactions, CPA2) (Henry et al. 1998; Dimitrova et al. 2004; Santos et al. 2010a). The IntEMGi for each of the 4 epochs were further corrected by the EMG integral of the baseline activity from −600 ms to −450 ms in relation to T0 as described below:
where IntEMGi is the integral of EMG activity of muscles inside each 150 ms epoch twi, i=1, ‥, 4, and is the 150 ms background muscle activity defined as the integral of EMG signal from −600 ms to −450 ms with respect to T0. Then integrals of EMG activity were normalized by the peak muscle activity.
This was done for each muscle for each subject. Due to the normalization, all the integral values (IEMGNORM) were within the range from +1 to −1 (Li and Aruin 2008). Positive values indicate an activation of the muscle, while negative values indicate a decrease in the background activity (inhibition).
Displacements of the center of pressure (COP) in the anterior-posterior direction were calculated using the following equation (Winter et al. 1996):
where Mx is the moment in sagittal plane, Fz and Fy are the vertical and the anterior-posterior components of the ground reaction force, and dz is the distance from the origin of the platform to the surface (0.038 m).
The kinematic data were first processed using the Vicon and BodyBuilder 3D modeling software. The PIG model consisted of fifteen body segments, including pelvis, femur (2), tibia (2), feet (2), humerus (2), radius (2), hands (2), thorax, and head. Body mass and height, 7 anthropometrical measures such as leg length, knee, ankle, elbow, and wrist width and shoulder offset and hand thickness for each subject were entered in the PIG model. These measures together with the kinematic data were used to calculate body’s COM position. All signals were then analyzed using MATLAB programs. Aligned trials were averaged for each subject. The displacement of COP and COM, at T0 which is anticipatory in nature and the peak displacement (maximum displacement after T0) that is compensatory in nature were calculated. As the perturbations were symmetrical, they were associated with anterior-posterior displacements of the COP and COM and negligible displacements in the medial-lateral direction. Therefore, COP and COM displacements in the anterior-posterior direction (Y-axis according to our experimental set up) will be reported.
It is important to note that the greater is the value of peak displacement during the compensatory postural control, the larger is the postural instability. The times at which the peak displacements occurred were also calculated for each of the above variables. These measures indicated the individuals’ balance control ability after the perturbations.
Statistical Analysis
Statistical analysis was performed in SPSS 17 for Windows XP (SPSS Inc., Chicago, USA). Means with standard errors are reported. For IEMGNORM, separate 2 × 4 split-plot ANOVAs were performed for each muscle. Group (2 levels: young and older adults) was a between subject factor and epoch (4 levels: APA1, APA2, CPA1, and CPA2) was a within-subject factor. When group × epoch interactions were significant, one-way ANOVA with Tukey’s Honestly Significant Difference (HSD) test was used for post hoc comparisons. To compare the latencies of individual muscles between the two groups, an independent t-test was performed. Independent t-tests were also performed for comparing displacements of COP and COM at T0 and at peak, and time of peak displacements between the two groups. Statistical significance was set at p < 0.05 for all tests.
Results
Muscle Latency
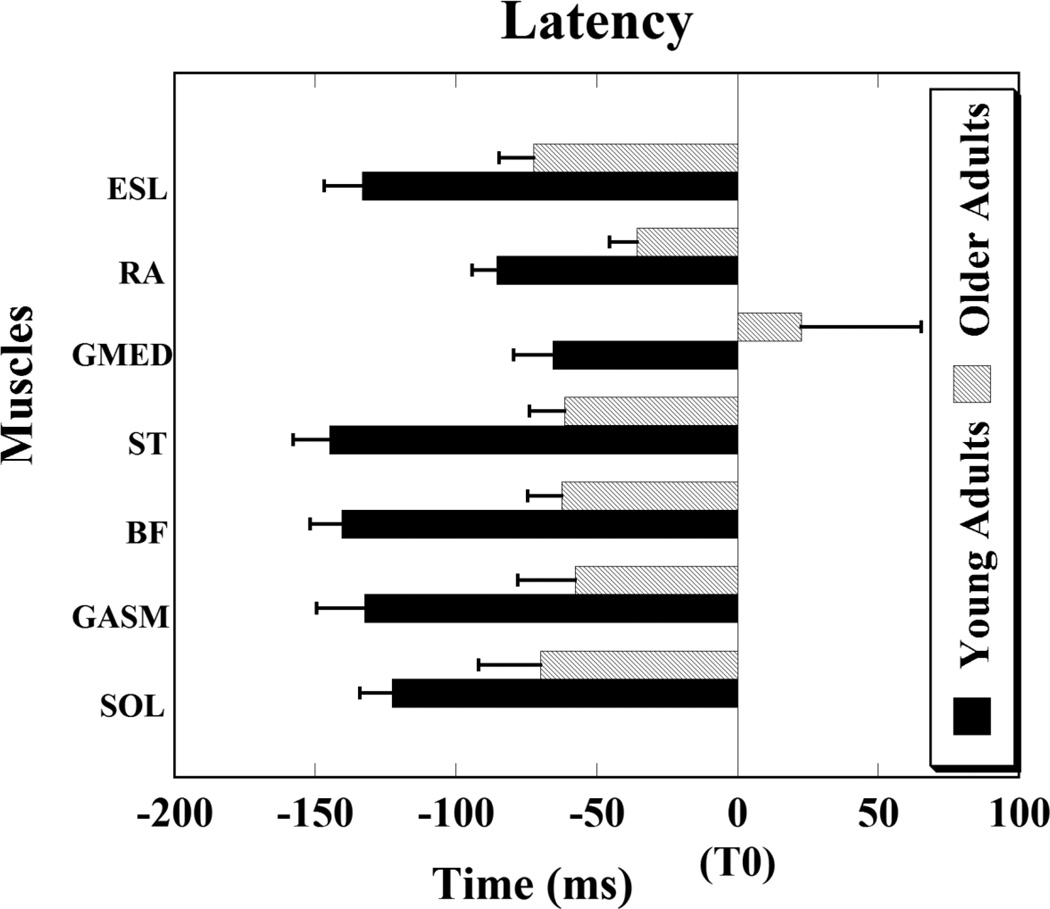
Overall, for all the thirteen muscles recorded, onset of anticipatory postural activity in older adults was delayed as compared to young adults. Thus, APA activity in older adults occurred close to the moment of perturbation and the difference between the two groups was statistically significant for seven muscles, namely: SOL, GASM, BF, ST, GMED, RA, and ESL (Fig. 2).
Figure 2.
Muscle latencies are shown for young and older adults. Note that for older adults the onset of muscle activity was delayed (close to the moment of perturbation (T0)) as compared to the young adults. Muscles abbreviations: SOL-soleus, GASM-medial gastrocnemius, BF- biceps femoris, ST-semitendinosis, GMED- gluteus medius, RA- rectus abdominis, and ESL- erector spine longus. Differences in latencies are significant for all muscles shown, p < 0.05.
The onset of APA activity for the two groups was as follows: SOL (young adults: −122.65 ± 11.43 ms, older adults: −69.96 ± 22.02 ms, p = 0.03); GASM (young adults: −132.33 ± 17.12 ms, older adults: −57.61 ± 20.48 ms, p = 0.01); BF (young adults: −140.48 ± 11.35 ms, older adults: −62.45 ± 12.02 ms, p < 0.001); ST (young adults: −144.91 ± 12.80 ms, older adults: −61.45 ± 12.38 ms, p < 0.001); GMED (young adults: −65.55 ± 13.90 ms, older adults: 22.66 ± 42.60 ms, p = 0.03); RA (young adults: −85.40 ± 8.81 ms, older adults: −35.69 ± 9.64 ms, p < 0.01); ESL (young adults: −133.31 ± 13.37 ms, older adults: − 72.36 ± 12.36 ms, p < 0.01). The order of activation of muscles for the young adults was from distal to proximal with a significant main effect for the muscle latency (F(6,71) = 4.72, p < 0.001). Post hoc analysis demonstrated that GASM, BF, and ST muscles had early APA onsets as compared to GMED muscle (p = 0.02, p = 0.006, p = 0.004, respectively). Moreover, BF and ST muscles showed early APA onsets as compared to RA muscle (p = 0.03, p = 0.01, respectively). Similar trend was observed in the older adults; however the differences between the muscle latencies were not statistically significant.
Integrated Electromyographic Activity
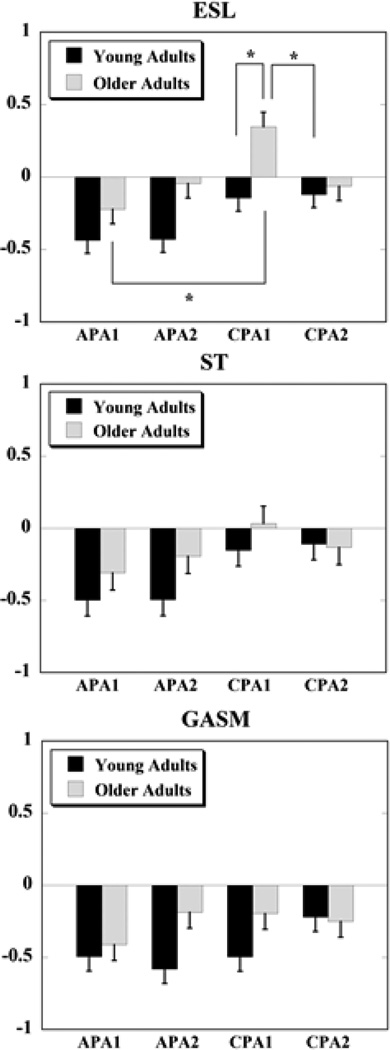
Overall, IEMGNORM data showed that compensatory muscle activity was larger in older adults as compared to young adults seen as larger increase or inhibition of muscle activity during the CPA1 and CPA2 phases. A significant main effect of group was seen for normalized integrals of EMG activity (IEMGNORM) in four muscles, namely: RF (F(1,21) = 8.05, p = 0.01), VM (F(1,20) = 9.83, p < 0.01), VL (F(1,21) = 4.81, p < 0.05), and ESL (F(1,21) = 9.69, p < 0.01). Young adults, on the other hand, had larger APAs and smaller CPAs. Specifically, the main effect of the group × epoch interaction was significant for GASM (F(3,63) = 3.57, p = 0.02), ST (F(3,63) = 2.89, p = 0.04), and was close to significance for ESL (F(3,63) = 2.74, p = 0.05) muscles (Fig. 3).
Figure 3.
Normalized integrated EMG activities during the four epochs (APA1, APA2, CPA1, and CPA2) are shown for the ESL (erector spinae longus), ST (semitendinosis), and GASM (medial gastrocnemius) muscles for young and older adults. Positive values indicate an activation of the muscle, while negative values indicate a muscle inhibition. * denotes p < 0.05.
Post-hoc analyses for the significant main interaction effects for GASM and ST muscles did not reveal any significant findings. However, an overall pattern was seen such that the APA1 and APA2 epochs were smaller while the CPA2 epoch was larger in older than young adults. The only exception to the overall pattern was for the CPA1 epoch which was smaller in older than young adults. For the ESL muscle (that showed a close to significance main interaction effect) post-hoc analysis demonstrated that the inhibitory activity during APA1 epoch was significantly smaller than the burst of muscle activity during the CPA1 epoch (p < 0.01) in older adults. Also, the APA1 and APA2 epochs were smaller in older adults than in young adults; however, the effects were not significant. Moreover, older adults had larger compensatory muscle activation during the CPA1 epoch than the inhibitory activity seen in young adults during the same epoch (p = 0.01). The CPA1 epoch in older adults was also significantly larger in magnitude than the CPA2 epoch in young adults (p = 0.02).
While there were differences in the magnitudes of anticipatory and compensatory activity between the older and young adults, both the groups used similar patterns of muscle activity in order to deal with the upcoming expected perturbation. Thus, in preparation for the perturbation (anticipatory phase), the dorsal muscles were mainly inhibited whereas the ventral and lateral muscles showed a burst of activity in both the groups. After the perturbation (compensatory phase), the dorsal muscles in both the groups continued with the inhibitory pattern except for the ESL muscle in the older adults group which showed a burst of activity during the CPA1 epoch and was then inhibited during the CPA2 epoch. The ventral and lateral muscles also continued with their burst of activity during the restoration phases in both the groups.
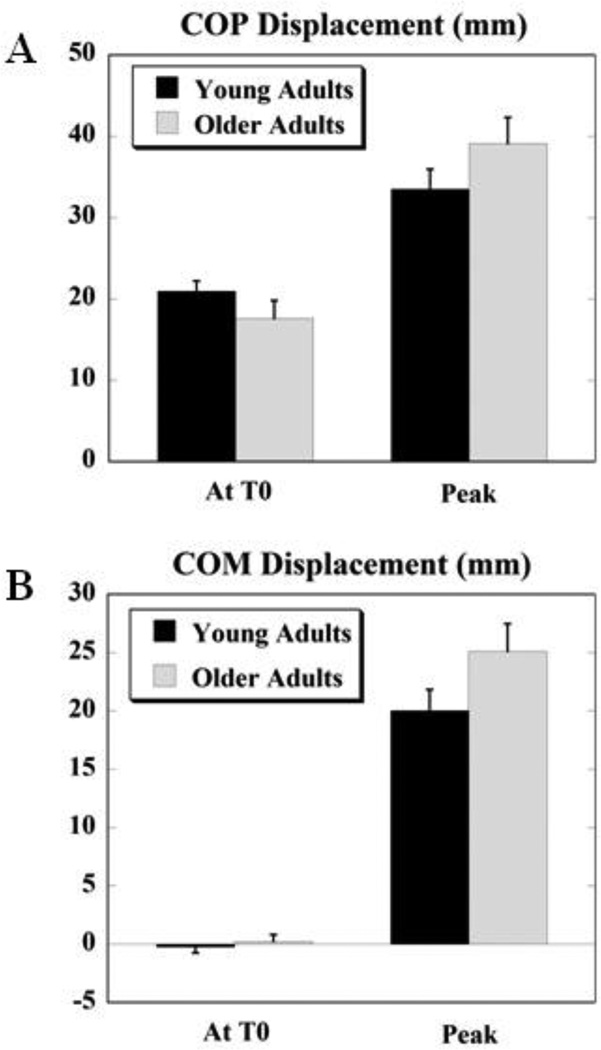
Displacements of Center of Pressure and Center of Mass
Fig. 4 depicts the displacements of COP (4A) and COM (4B) respectively, at T0 and the peak displacements after T0 for the two groups. The differences in COP and COM displacements at T0 and the peak displacements were not significant between the two groups. The overall pattern was such that, the anticipatory COP displacement (at T0) in older adults (17.62 ± 2.21 mm) was smaller than that in young adults (20.99 ± 1.25 mm) whereas, the peak displacement was larger in older adults (39.12 ± 3.22 mm) than young adults (33.51 ± 2.45 mm). Moreover, there was a significant negative correlation between the COP displacement at T0 and the peak COP displacement for the older adults (r = − 0.83, p = 0.03). The COP moved in the posterior direction (i.e. in the direction of perturbation) prior to the perturbation and continued moving in the same direction after the perturbation for both, young and older adults.
Figure 4.
A. Displacement of COP at T0 (anticipatory) and peak displacement (after T0, compensatory) for young and older adults. B. Displacement of COM at T0 (anticipatory) and peak displacement (after T0, compensatory) for young and older adults. Positive values indicate displacement in the posterior/backward direction and negative values indicate displacement in the anterior/forward direction.
The anticipatory COM displacement was of similar magnitude between the two groups. However, while the COM moved forwards (i.e. opposite to the direction of perturbation) in preparation for the impact in young adults, it moved backwards (i.e. in the direction of perturbation) in older adults. The peak COM displacement showed a trend of greater magnitude for the older adults (25.13 ± 2.36 mm) as compared to the young adults (20.04 ± 1.79 mm) and the COM moved backwards (i.e. in the direction of perturbation) after the impact in both the groups. Moreover, there was a significant positive correlation between the COM displacement at T0 and the peak COM displacement for the young (r = 0.60, p = 0.03) and older adults (r = 0.88, p < 0.01).
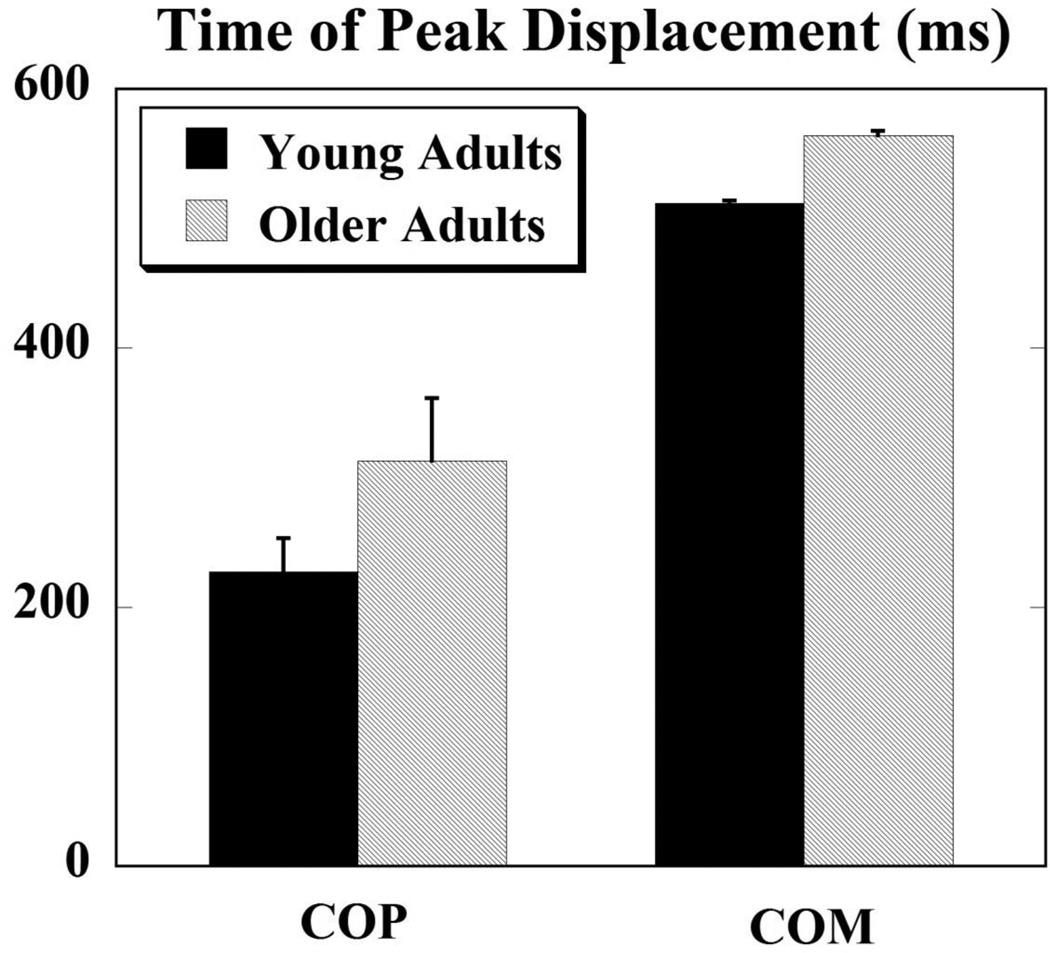
The COP was the first to reach its peak after the perturbation for both the groups. The time when the COP and COM variables reached their peaks was not significantly different between the young and older adults. The time of the peak COP displacement for young adults was 227.11 ± 26.28 ms and for older adults it was 313.14 ± 48.54 ms after T0. The COM reached its peak after the COP; its times were 511.6 ± 2.46 ms for young adults and 564.2 ± 3.30 ms after T0 for older adults (Fig. 5).
Figure 5.
Times at which COP and COM reached their peak displacements after the perturbation for young and older adults.
Discussion
This study was focused on investigating the differences in anticipatory postural adjustments between young and older adults and its effect on subsequent control of posture. Overall, the study hypothesis was supported such that as compared to young adults, APAs in older adults were delayed resulting in larger CPAs and greater peak COP and COM displacements after the perturbation.
APAs in the elderly: role of self-initiated and external perturbations
Previous studies have used self-generated perturbations to study anticipatory postural activity in older adults. Overall, a delay in anticipatory postural activity was seen in older adults prior to the movement onset; however, several studies also found a delay in the onset of movement as well smaller peak accelerations and prolonged movement times (Man'kovskii et al. 1980; Inglin and Woollacott 1988; Rogers et al. 1992; Woollacott and Manchester 1993; Garland et al. 1997). Investigations involving the arm raising paradigm performed under different velocities have shown that older adults maintain arm velocities comparable to young adults (Woollacott and Manchester 1993; Bleuse et al. 2006), thus concluding that any alterations in APAs could be attributed to the direct effects of aging and velocity is not a mediating variable between age and lack of postural preparation (Woollacott and Manchester 1993). In addition, a delay only in the onset of postural muscle response was observed with no changes in the onset of the focal muscle.
On the other hand, when older individuals were asked to perform focal movements at a comfortable slow speed, a strong correlation between postural activity and focal movement existed and they performed the motor task confidently (Man'kovskii et al. 1980). However, such correlation decreased when movements were required to be performed rapidly, leading to deterioration of performance of the motor task. It was speculated that given these findings, older individuals may perform motor tasks advantageously slowly so as to achieve optimal postural preparation. Also, studies demonstrating similar movement performance between the elderly and younger groups found that despite this result, differences existed across muscle and velocity conditions between the two groups (Woollacott and Manchester 1993). This indicates that some effect of voluntary movement performance due to aging may still exist.
These results thus raised an important question of whether the changes in APAs seen in the elderly are due to the direct effect of aging or does the aging process slow down voluntary movements, which in turn affects APAs. The inconsistencies in previous findings could be attributed to different testing paradigms (push and pull tasks, arm raising performed under self-paced or reaction time conditions) and measurement outcomes (onset latencies, movement times, arm velocity or acceleration). In order to clearly understand the effect of aging on the organization of APAs, it is essential to discern the influence of voluntary movement performance from postural preparation. To this end, the outcome of the present study using the pendulum-impact paradigm (which generates external predictable perturbations of constant magnitude) showed the direct effect of aging on anticipatory postural activity without the influence of age-related changes in voluntary movement performance.
Age-related changes in anticipatory postural control
In the present study, anticipatory muscle activity was significantly delayed in older adults as compared to young adults, such that the muscles were either activated or inhibited very close to the moment of perturbation. In fact, the GMED muscle was activated only after the impact, although the perturbation was predictable. Similarly, previous studies have demonstrated that APA activity associated with self generated body perturbations is significantly delayed in the healthy elderly, with postural muscles being recruited closer to the activation of prime mover muscles (Man'kovskii et al. 1980; Inglin and Woollacott 1988; Rogers et al. 1992; Woollacott and Manchester 1993) or after prime mover activation (Woollacott and Manchester 1993). Moreover, the delayed anticipatory muscle activity was associated with large magnitudes of compensatory muscle activity (during CPA1 epoch) in older than young adults.
Delayed anticipatory muscle activity in older adults was also associated with smaller anticipatory COP displacements, while the anticipatory COM displacement was of similar magnitude between the two groups in this study. This resulted in larger peak displacements of both, the COP and COM, in older adults after the perturbation, indicating their greater instability. Likewise, a previous study reported delay in the anticipatory vertical torque (Tz) was observed in the elderly performing arm raising task at maximal velocity (self-paced with and without load and externally triggered), whereas COP latency was reduced only in the self-paced condition without load (Bleuse et al. 2006)
Moreover, in the present study, while the COM moved forwards (i.e. opposite to the direction of perturbation) in preparation for the impact in young adults, it moved backwards (i.e. in the direction of perturbation) in older adults. Such impaired directional control in older adults, wherein the anticipatory movement of the COM was in the direction of the eventual perturbation, may have in fact contributed to the larger COM displacement after the disturbance. Thus, while the older adults had similar magnitude of anticipatory COM movement, it was not directionally specific, thereby possibly augmenting the instability.
The findings of this study have demonstrated that aging does directly affect the organization (muscle onset and magnitude) of anticipatory postural control in humans. Additionally, inadequate postural preparation in the elderly is associated with a need for larger compensatory muscle activity which may not be sufficient to overcome the impact of large perturbations. Therefore, impaired postural preparation comes with a potential for loss of balance in older adults.
It is important to note that although APA activity was delayed in older adults in the present study, all muscles except GMED showed at least some anticipatory muscle activity. Elderly subjects have been found to recruit their lower limb and trunk muscles prior to a focal movement as frequently as (96–100% of trials) the young subjects (Rogers et al. 1992). While delayed onset of APA muscle activity is observed in older adults, aging does not seem to affect recruitment of postural muscles in a feedforward manner as has been demonstrated in some of the previous studies (Rogers et al. 1992; Garland et al. 1997; Bleuse et al. 2006). Furthermore, older adults are capable of generating appropriate patterns of anticipatory and compensatory muscle activity (similar to those seen in young adults) that are directionally specific and useful in dealing with the perturbation effects. Thus, it seems that deficits in postural control with aging do not appear to be due to a complete absence of APAs, which have otherwise been found to be sometimes absent in neurological conditions of the aged such as Parkinson’s disease (Bazalgette et al. 1987).
Some attempts have made to explain the observed changes in anticipatory postural adjustments of the elderly. Thus, an analysis of neurological impairments in the older subjects demonstrated a strong correlation between those adults showing sub-clinical neural deficits and those with abnormal muscle response characteristics (Woollacott and Manchester 1993). Findings of a recent study on APAs related to predictive perturbations and concurrent cognitive task, suggested that postural control seems to be less automated in the elderly and becomes insufficient during very challenging perturbations (Laessoe and Voigt 2007). Age related morphological and biochemical changes are known to occur in the neural structures implicated in preparatory postural adjustments (Massion 1992). Specifically, age-related impairments in structures that are implicated as neural detectors of postural instability, such as the supplementary motor area and the foot area of the sensorimotor cortex (Slobounov et al. 2005), may be responsible for greater postural instability seen in the older adults. Therefore, the deviations in the postural muscle responses in the elderly seen in the present study may reflect age related modifications in the central nervous system structures controlling posture.
Conclusion
The process of aging affects an individual’s ability to control posture putting him/her at an increased risk of suffering falls. Particularly anticipatory postural control which helps deal with body perturbations in a feedforward manner has been found to be affected in the elderly. This study demonstrated that anticipatory postural adjustments are delayed in older adults as compared to young adults. Impaired generation of APAs is associated with larger compensatory muscle responses in older adults. However, in spite of such larger reactive responses, older adults are still more unstable than young adults when exposed to similar perturbations. Nonetheless, while APAs are impaired in older adults, the ability to recruit muscles anticipatorily is largely preserved, however, due to delayed onsets, it is likely that their effectiveness in reducing the magnitude of CPAs is smaller. As such, there is potential that training-related improvements in APAs of older adults may be beneficial in optimizing the magnitudes of compensatory muscle responses. The outcome of this study, lends support towards investigating the role of training in improving anticipatory postural control in people with balance impairments due to aging or neurological disorders.
Acknowledgements
This work was supported in part by the NIH grant # HD064838. We also thank Vennila Krishnan for help in data collection and Xiaoyan Li for assistance in data processing.
References
- Alexandrov AV, Frolov AA, Horak FB, Carlson-Kuhta P, Park S. Feedback equilibrium control during human standing. Biol Cybern. 2005;93:309–322. doi: 10.1007/s00422-005-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res. 1995;106:291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Shiratori T, Latash ML. The role of action in postural preparation for loading and unloading in standing subjects. Exp Brain Res. 2001;138:458–466. doi: 10.1007/s002210100729. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Blumenstein R. Electrode placement in EMG biofeedback. Baltimore: Williams & Wilkins; 1980. [Google Scholar]
- Bazalgette D, Zattara M, Bathien N, Bouisset S, Rondot P. Postural adjustments associated with rapid voluntary arm movements in patients with Parkinson's disease. Adv Neurol. 1987;45:371–374. [PubMed] [Google Scholar]
- Belen'kii VE, Gurfinkel VS, Pal'tsev EI. Control elements of voluntary movements. Biofizika. 1967;12:135–141. [PubMed] [Google Scholar]
- Bleuse S, Cassim F, Blatt JL, Labyt E, Derambure P, Guieu JD, Defebvre L. Effect of age on anticipatory postural adjustments in unilateral arm movement. Gait Posture. 2006;24:203–210. doi: 10.1016/j.gaitpost.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech. 1987;20:735–742. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Horak FB, Nutt JG. Postural muscle responses to multidirectional translations in patients with Parkinson's disease. J Neurophysiol. 2004;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- Frank JS, Patla AE. Balance and mobility challenges in older adults: implications for preserving community mobility. Am J Prev Med. 2003;25:157–163. doi: 10.1016/s0749-3797(03)00179-x. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly, and hemiplegic subjects. Arch Phys Med Rehabil. 1997;78:1072–1077. doi: 10.1016/s0003-9993(97)90130-1. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. EMG responses to maintain stance during multidirectional surface translations. J Neurophysiol. 1998;80:1939–1950. doi: 10.1152/jn.1998.80.4.1939. [DOI] [PubMed] [Google Scholar]
- Hess JA, Woollacott MH, Shivitz N. Ankle force and rate of force production increase following high intensity strength training in frail older adults. Aging Clinical and Experimental Research. 2005;18:107–115. doi: 10.1007/BF03327425. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Inglin B, Woollacott M. Age-related changes in anticipatory postural adjustments associated with arm movements. J Gerontol. 1988;43:M105–M113. doi: 10.1093/geronj/43.4.m105. [DOI] [PubMed] [Google Scholar]
- Laessoe U, Voigt M. Anticipatory postural control strategies related to predictive perturbations. Gait Posture. 2007 doi: 10.1016/j.gaitpost.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Li X, Aruin AS. Anticipatory postural adjustments in conditions of simulated reduced gravity. Gait Posture. 2008;28:538–544. doi: 10.1016/j.gaitpost.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Man'kovskii NB, Mints A, Lysenyuk VP. Regulation of the preparatory period for complex voluntary movement in old and extreme old age. Hum Physiol. 1980;6:46–50. [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Mohapatra S, Aruin AS. Static and dynamic visual cues in feed-forward postural control. Exp Brain Res. 2013;224:25–34. doi: 10.1007/s00221-012-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S, Krishnan V, Aruin AS. The effect of decreased visual acuity on control of posture. Clin Neurophysiol. 2012;123:173–182. doi: 10.1016/j.clinph.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashner LM, Cordo PJ. Relation of automatic postural responses and reaction-time voluntary movements of human leg muscles. Exp Brain Res. 1981;43:395–405. doi: 10.1007/BF00238382. [DOI] [PubMed] [Google Scholar]
- Park S, Horak FB, Kuo AD. Postural feedback responses scale with biomechanical constraints in human standing. Exp Brain Res. 2004;154:417–427. doi: 10.1007/s00221-003-1674-3. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Soderberg GL. Age-related changes in postural responses preceding rapid self-paced and reaction time arm movements. J Gerontol. 1992;47:M159–M165. doi: 10.1093/geronj/47.5.m159. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Role of lateral muscles and body orientation in feedforward postural control. Exp Brain Res. 2008;184:547–559. doi: 10.1007/s00221-007-1123-9. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010a;20:388–397. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J Electromyogr Kinesiol. 2010b;20:398–405. doi: 10.1016/j.jelekin.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori T, Latash ML. Anticipatory postural adjustments during load catching by standing subjects. Clin Neurophysiol. 2001;112:1250–1265. doi: 10.1016/s1388-2457(01)00553-3. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Beyer N. Exercise and injury prevention in older people. Scand J Med Sci Sports. 2003;13:77–85. doi: 10.1034/j.1600-0838.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Hallett M, Stanhope S, Shibasaki H. Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol. 2005;116:315–323. doi: 10.1016/j.clinph.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Manchester DL. Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? J Gerontol. 1993;48:M64–M70. doi: 10.1093/geronj/48.2.m64. [DOI] [PubMed] [Google Scholar]