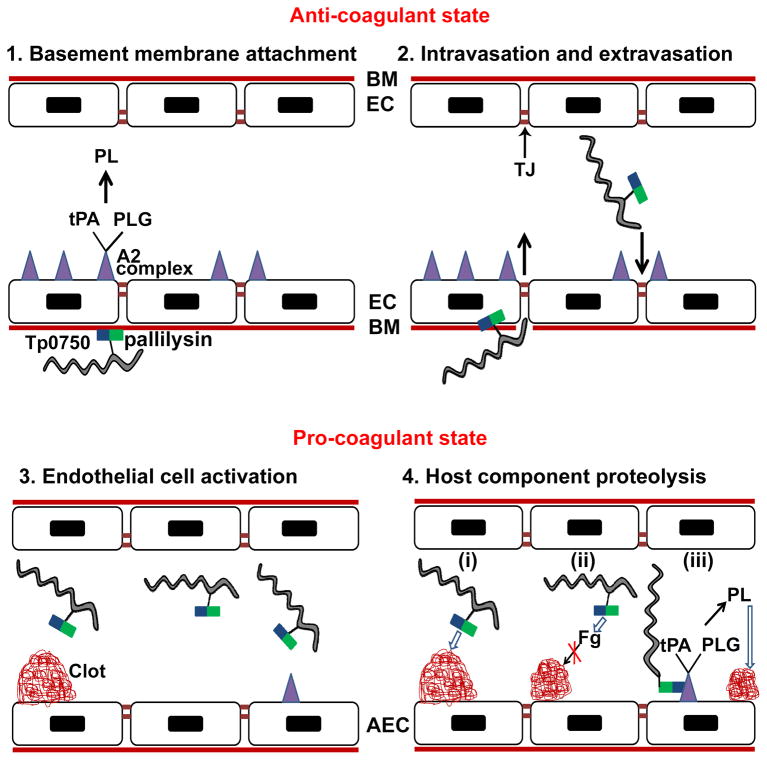
Fig. 8. Proposed model of the role of Tp0750/pallilysin in T. pallidum dissemination via the bloodstream.
(1) Surface-exposed pallilysin (
 ) mediates attachment of Treponema pallidum (
) mediates attachment of Treponema pallidum (
 ) to the laminin-rich basement membrane (BM) that underlies the endothelial cells (EC) of blood vessels. The unperturbed endothelium exists primarily in an anti-coagulant state due to the expression of profibrinolytic factors including the fibrinolytic complex comprising annexin A2 and S100A10 (
) to the laminin-rich basement membrane (BM) that underlies the endothelial cells (EC) of blood vessels. The unperturbed endothelium exists primarily in an anti-coagulant state due to the expression of profibrinolytic factors including the fibrinolytic complex comprising annexin A2 and S100A10 (
 ) which binds tissue plasminogen activator (tPA) and plasminogen (PLG) resulting in plasmin (PL)-mediated clot dissolution; (2) T. pallidum intravasation and extravasation: pallilysin degrades the basement membrane. Tp0750 (
) which binds tissue plasminogen activator (tPA) and plasminogen (PLG) resulting in plasmin (PL)-mediated clot dissolution; (2) T. pallidum intravasation and extravasation: pallilysin degrades the basement membrane. Tp0750 (
 ), through interaction with annexin A2, localizes T. pallidum to areas of tight junction (TJ) remodeling; (3) Activation of endothelial cells occurs upon infection with T. pallidum leading to the formation of a pro-coagulant endothelial surface. On the surface of activated endothelial cells (AEC), the coagulation cascade is initiated resulting in the generation of vascular clots composed of fibrin, fibronectin, and platelets; (4) Tp0750/pallilysin-mediated host component proteolysis: (i) Direct clot degradation via pallilysin-mediated fibrinolysis and Tp0750-mediated fibronectinolysis, (ii) clot formation inhibition via Tp0750/pallilysin-mediated fibrinogen (Fg) degradation, and (iii) Tp0750-annexin A2 interaction localizes T. pallidum to the immediate vicinity of plasmin-mediated vascular fibrinolysis. Open arrows indicate direct proteolysis of host components. Closed arrows indicate an intermediate pro-protease activation step prior to direct proteolysis of host components.
), through interaction with annexin A2, localizes T. pallidum to areas of tight junction (TJ) remodeling; (3) Activation of endothelial cells occurs upon infection with T. pallidum leading to the formation of a pro-coagulant endothelial surface. On the surface of activated endothelial cells (AEC), the coagulation cascade is initiated resulting in the generation of vascular clots composed of fibrin, fibronectin, and platelets; (4) Tp0750/pallilysin-mediated host component proteolysis: (i) Direct clot degradation via pallilysin-mediated fibrinolysis and Tp0750-mediated fibronectinolysis, (ii) clot formation inhibition via Tp0750/pallilysin-mediated fibrinogen (Fg) degradation, and (iii) Tp0750-annexin A2 interaction localizes T. pallidum to the immediate vicinity of plasmin-mediated vascular fibrinolysis. Open arrows indicate direct proteolysis of host components. Closed arrows indicate an intermediate pro-protease activation step prior to direct proteolysis of host components.