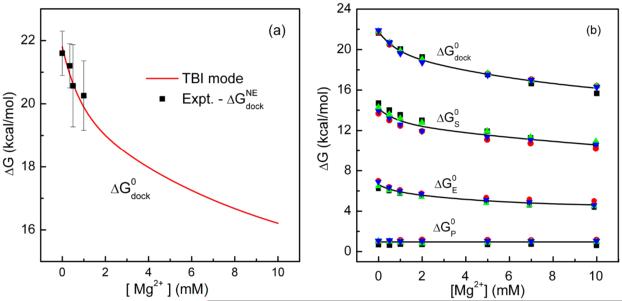
Figure 3.
The total docking free energy ΔGdock and its components. (a) Comparison between the predicted electrostatic free energy for docking ΔGdock and the experimental data for the total docking free energy minus 20.6 kcal/mol.22 Here the non-electrostatic contribution () of −20.6 kcal/mol accounts for the tertiary interaction energy between the tetraloop and the receptor. (b) The total docking free energy (ΔGdock) and the corresponding component free energies as a function of the Mg2+ concentration in the background of 0.1M NaCl at 37°C. ΔGS, ΔGE and ΔGP are respectively the entropic free energy, the Coulomb energy and the solvent polarization energy contributions.