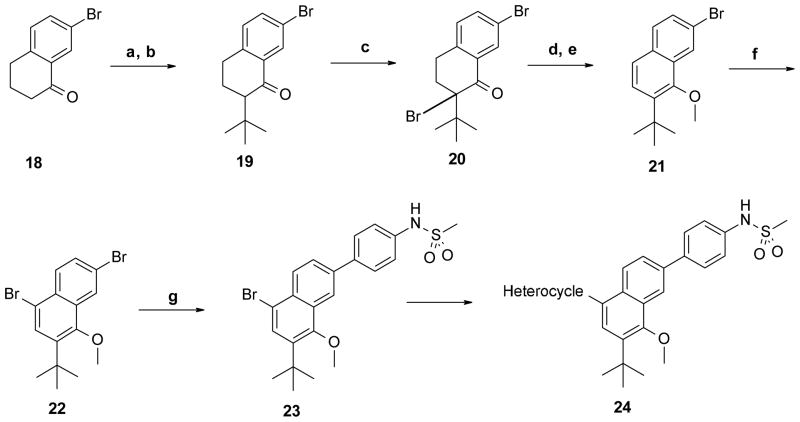
Scheme 3. Synthesis of 2,5,7,8-tetrasubstituted naphthalene core.
Reagents and conditions: (a) TMSCl, NaI, Et3N, CH3CN, 69%; (b) 2-chloro-2-methyl-propane, TiCl4, CH2Cl2, −40 °C, 50%; (c) Br2, AcOH, 50 °C, 98%; (d) LiBr, Li2CO3, DMF, 100 °C; (e) MeI, K2CO3, DMF, 96% two steps; (f) Br2, AcOH, 98%; (g) 4-(methanesulfonamido)phenylboronic acid, Pd(PPh3)4, Na2CO3, MeOH, toluene, 115 °C, 54%.