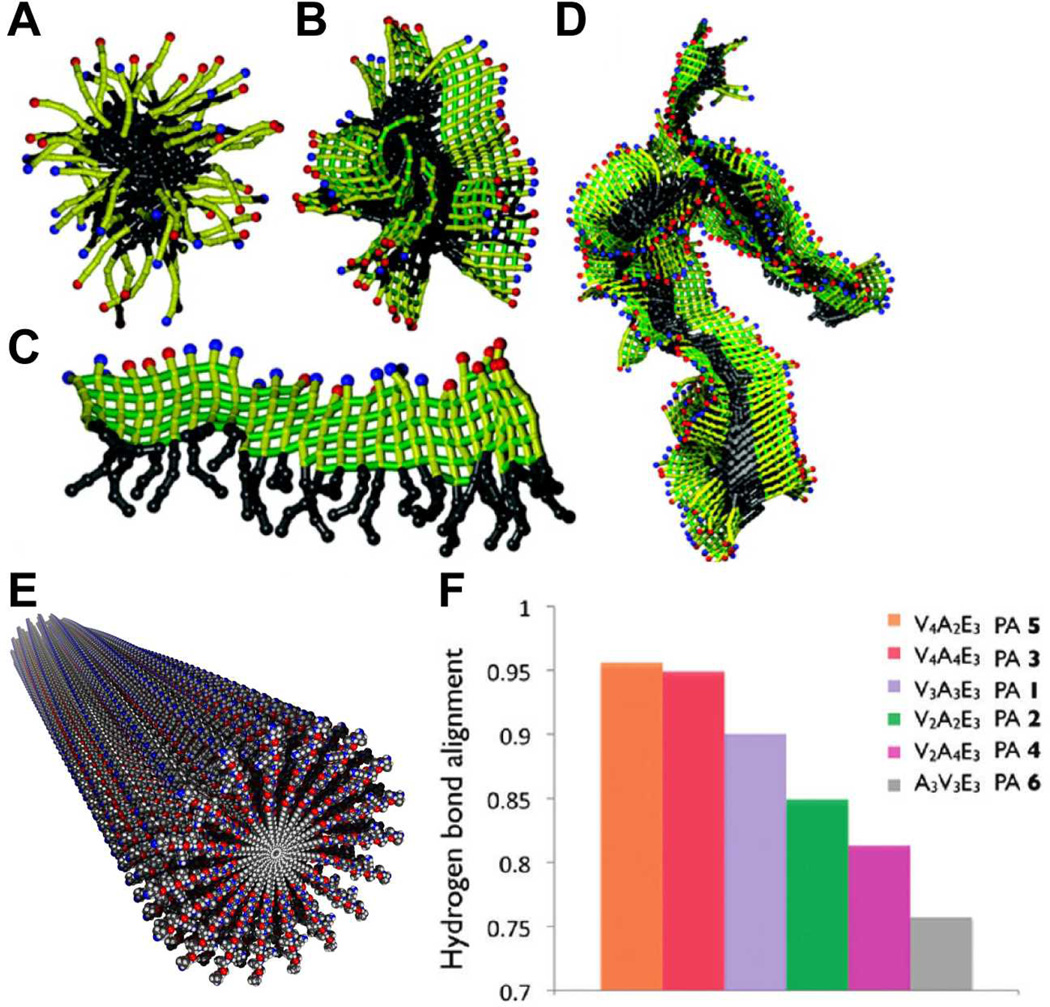
Fig. 3.
(A–D) Snapshots from molecular simulations of peptide amphiphiles with increasing degrees of hydrogen bonding strength in their β domains. (A) A spherical micelle forms through hydrophobic collapse; (B) a more elliptical micelle is predicted as β-sheets form; (C) an extended supramolecular aggregate formed by an extended β-sheet of peptide amphiphiles; (D) aggregating β-sheets through hydrophobic interactions. (E) molecular graphics representation of a cylindrical nanofiber in which β-sheets are twisted about its long axis. (F) The degree of twist decreases with increasing hydrogen bond alignment, which is controlled by the first amino acids adjacent to the alkyl tail. (A–D) are adapted with permission from reference 41. Copyright 2010 American Chemical Society. (F) is adapted with permission from reference 46. Copyright 2010 American Chemical Society.