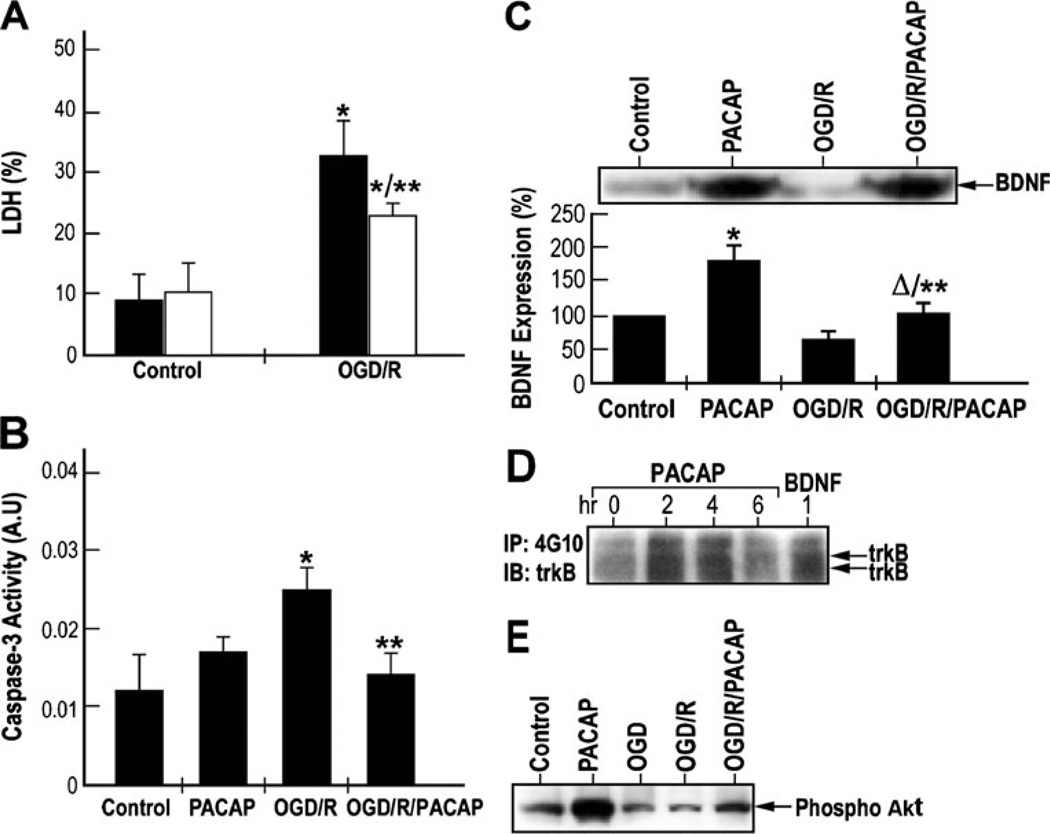
Fig. 6.
PACAP induced neuroprotection, BDNF release, and trkB and Akt phosphorylation in cultured cortical neurons subjected to oxygen–glucose deprivation (OGD). a Neuroprotective effects of PACAP on OGD-induced neuronal cell death in vitro. Cultured cortical neurons were subjected to OGD for 1 h followed by reperfusion for 48 h, in the absence (OGD/R) or presence (OGD/R/PACAP) of 100 nM of PACAP38. The levels of released and total lactate dehydrogenase (LDH) were measured by using a CytoTox 96 cytotoxicity assay kit. Results were presented as mean±SD, *p<0.05 compared to control; **p<0.05 compared to the saline group exposed to oxygen–glucose deprivation followed by reoxygenation (OGD/R) (black—saline; white —PACAP38). b Inhibitory effects of PACAP on caspase-3 activity under OGD followed by reperfusion. Cultured cortical neurons were subjected to OGD for 1 h followed by reperfusion as in a. Caspase-3 activity was measured using caspase-3 assay kit. Results were presented as mean±SD. *p <0.05 compared to control; **p <0.05 compared to OGD/R. c Upper—western blotting analysis of BDNF expression. Lower—quantization of BDNF levels using ELISA. *p< 0.001 compared to control; **p<0.001 compared to OGD/R; Δp<0.05 compared to PACAP. d Effects of PACAP on the activation of trkB-mediated signaling pathways in cultured cortical neurons. Cultured cortical neurons were treated with 100 nM of PACAP38 for 2, 4, and 6 h. Cells were also treated with 50 ng/ml of BDNF for 1 h. Equal amounts of lysates were immunoprecipitated with anti-phosphotyrosine antibody and immunoblotted with anti-trkB or anti-PI3-K p85 antibodies. e PACAP induces Akt phosphorylation under ischemic conditions. Cultured cortical neurons were subjected to OGD for 1 h followed by reperfusion for 48 h, in the absence or presence of 100 nM of PACAP38. Equal amounts of lysates were subjected to western blotting analysis. Phosphorylation of Akt was detected by using an anti-phospho-Akt antibody (ser 43)