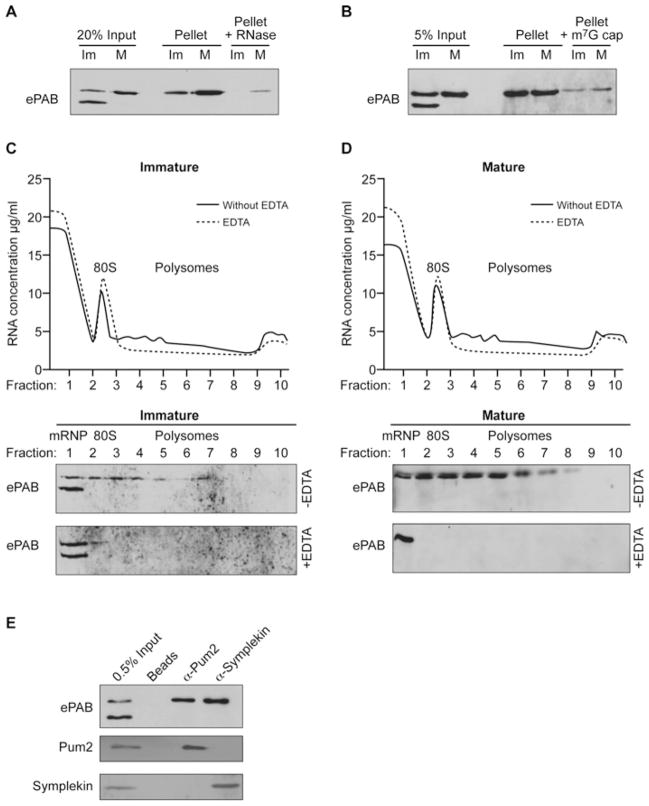
Figure 3. Hyperphosphorylated ePAB is associated with mRNA translation and cytoplasmic polyadenylation complexes.
(A) Poly(A) RNA was selected from immature (Im) or in vitro matured (M) oocytes using oligo(dT) cellulose (pellet) and was Western blotted for co-isolated ePAB. RNA-dependence was established by RNase I pre-treatment (Pellet + RNase). (B) Western blot of ePAB isolated by m7GTP-Sepharose chromatography (Pellet) from lysates (Input) of immature (Im) or mature (M) oocytes. Excess m7G cap analogue (~100-fold) reduces ePAB-association with the resin (Pellet + m7G cap). (C) Lysates derived from immature oocytes were separated over a 10–40 % sucrose gradient, and the positions of the 80S peak, polyribosomes (Polysomes) and messenger ribonucleoproteins (mRNPs) are indicated (upper panel). EDTA disrupts polyribosomes and was omitted (without EDTA) or added (EDTA) to lysates. The resulting fractions were Western blotted for ePAB (lower panels). (D) As for (C) except that matured oocytes were used. (E) Lysate (Input) from immature oocytes was incubated with anti-Pum2 or anti-Symplekin antibodies or beads alone (Beads) as indicated, and co-precipitating proteins were detected by immunoblotting.