Fig. 1.
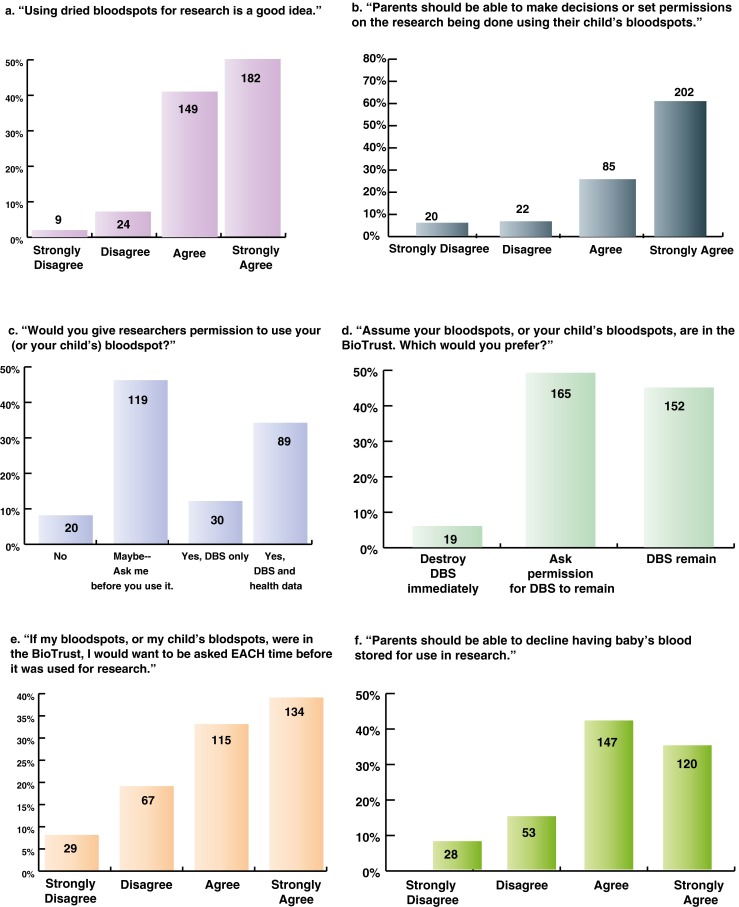
Consent preferences. Overall, 91 % of community members agreed that using DBS for research is a good idea, while 87 % felt that “parents should be able to make decisions or set permissions on the research being done using their child’s bloodspots.” Forty-six percent of participants said they would give researchers permission to use their or their child’s DBS with or without health data, while 20 % said they would not, and 46 % responded “maybe: ask me before you use it.” A minority of participants (6 %) said they would want to have their or their child’s DBS destroyed if they were in the BioTrust; 45 % said they would want them to remain, and 49 % said they would want to be asked permission for them to remain. Seventy-two percent of participants said they would want to be asked each time their or their child’s DBS would be used for research. Twenty-three percent of participants indicated they felt that parents should not be able to “decline having baby’s blood stored for use in research”
