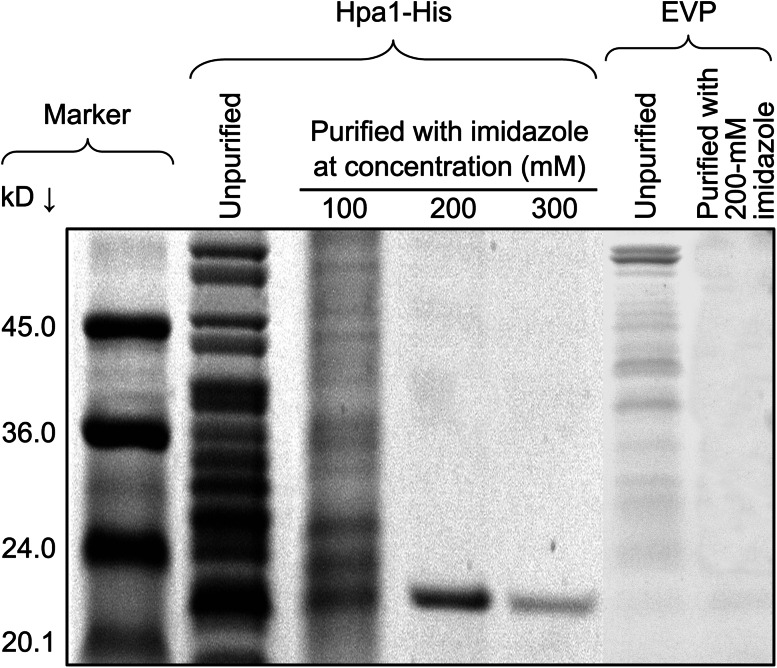
Fig. 1.
Protein electrophoresis analysis. The Hpa1-His fusion protein was produced through a recombinant prokaryotic vector and analyzed in comparison with the empty vector preparation (EVP) that did not contain Hpa1-His. Protein samples without purification were analyzed directly by electrophoresis on the tricine-sodium dodecylsulphate-plolyacrylamide gel. Alternatively, protein samples were bound to nickel-polystyrene beads, eluted with the indicated concentrations of imidazole, and then analyzed by electrophoresis. Protein bands were visualized by gel staining with Coomassie G-250. Molecular makers are indicated. The Hpa1-His fusion protein from the 200-mM imidazole eluent was treated with an enterokinase cleavage capture reagent to remove the His tag and only Hpa1 was used in plant treatment. The 200-mM imidazole eluent of the EVP preparation was used as a negative control