Abstract
The median raphe nucleus (MR) has been shown to exert a powerful influence on behavioral arousal and marked locomotor hyperactivity can be produced by intra-MR injections of a variety of drugs including GABAA and GABAB agonists, excitatory amino acid antagonists, and μ- and δ-opioid agonists. Other studies have indicated that the MR exerts an inhibitory influence on ascending dopamine systems, suggesting that MR induced alterations in activity may be mediated through changes in dopaminergic transmission. In the present study, we explored this possibility by examining whether systemic administration of the preferential D2 dopamine antagonist haloperidol is able to antagonize the hyperactivity produced by intra-MR injections of various drugs. We found that haloperidol completely blocked the locomotor response to intra-MR injections of the μ-opioid receptor agonist DAMGO and the δ-opioid receptor agonist DPDPE. In marked contrast, at doses which abolished the locomotor response to systemic amphetamine, haloperidol had no effect on the hyperactivity induced by intra-MR injections of GABAA agonist muscimol, the GABAB agonist baclofen, or the kainate/quisqualate antagonist pBB-PZDA, even though it suppressed baseline activity in these same animals. These results indicate that there must be at least two mechanisms capable of influencing behavioral arousal within the MR region, one of which is dependent on D2 dopamine receptors and the other not.
Keywords: locomotion, hyperlocomotion, tegmentum, nucleus centralis superior, dopaminergic, RMTg
The mesencephalic median raphe nucleus (MR) is known to exert a major influence on locomotor activity. For example, marked increases in locomotion are seen after either electrolytic or excitotoxic lesions of the MR [1–5], and even more dramatic effects can be observed after intra-MR injections of a variety of drugs, including GABAA and GABAB agonists, excitatory amino acid (EAA) antagonists, and μ- and δ-opioid agonists [6–10]. These responses are, in general, much larger when the drugs are injected into the MR than when they are applied to surrounding structures [6–8,11], suggesting that their effects are exerted within the immediate vicinity of the MR, rather than through diffusion to distant structures. Even though the MR is one of the major sources of serotonergic projections to the forebrain, the majority of cells in this nucleus utilize transmitters other than serotonin [12], and several lines of evidence indicate that influence of the MR on activity is largely mediated through nonserotonergic mechanisms [2,9,13].
While the pathways through which the MR exerts its dramatic effects on locomotion remain to be identified, one interesting possibility is suggested by the observation that the MR sends a projection to the ventral tegmental area (VTA) [14–16], the origin of ascending dopaminergic projections to the nucleus accumbens and olfactory tubercle. Further, it has been shown that dopamine turnover in the nucleus accumbens can be increased by intra-MR injections of GABAA, GABAB, μ- and δ-opioid receptor agonists, as well as by injections of EAA antagonists [6–8,17,18]. Since stimulation of dopamine receptors within the accumbens is well known to increase locomotor activity [19,20], it seems plausible that the effects of these various drugs injected into the MR might be mediated through their common effects on dopamine release. We previously explored this possibility with respect to the hyperactivity produced by injections of the GABAA agonist muscimol by examining whether the response to this drug could be antagonized by systemic injections of the preferential D2 dopamine receptor antagonist haloperidol [17]. We found, however, that haloperidol was without significant effect on muscimol-induced hyperactivity, even when the dopamine antagonist was given at doses which, by themselves, produced marked akinesia and catalepsy.
Since haloperidol is able to completely antagonize the hyperactivity induced by dopamine releasing agents, these findings suggest that the effects of intra-MR muscimol injections must be largely independent of changes in dopamine release. It is not known, however, whether a similar conclusion holds for the responses seen after injections of other drugs into the MR. In the current study we therefore investigated the effects of systemic haloperidol injections on the responses to intra-MR injections of the GABAB agonist baclofen, the μ-opioid agonist D-Ala-Gly-MePhe-Gly(ol)-enkephalin (DAMGO), the δ-opioid agonist D-pen2,D-Pen5-enkephalin (DPDPE), and the preferential AMPA/kainate antagonist p-bromobenzoyl-piperazine-2,3-dicarboxylate (pBB-PZDA). In order to confirm our previous findings [17], we also examined animals treated with intra-MR muscimol. Finally, in order to compare effects on MR mediated changes in locomotion to those on a response known to be mediated by dopamine receptor stimulation, we examined the effects of haloperidol on the hyperactivity produced by systemic injections of d-amphetamine. The results of these experiments suggest that dopamine is differentially involved in the responses to different drugs injected into the MR.
METHODS
Subjects
Subjects were 38 adult, male Sprague-Dawley derived rats weighing 280–320g obtained from the colony of the University of Illinois at Chicago. Rats were housed individually on a 12:12 hour light:dark cycle in wire-mesh cages with food (Purina Lab Chow) and water available ad libitum. Thirty-two animals received intracranial cannulae aimed at the MR, whereas the remaining 6 subjects, who were used to examine the effects of systemic amphetamine, were not operated.
Surgery
Rats were anesthetized with sodium pentobarbital (50mg/kg). Using standard aseptic technique, 22-gauge guide cannulae aimed to terminate 2 mm above the center of the MR (AP:−0.3: L;0.0: H;−2.3, mm from the midpoint of the interaural line[21]) were implanted stereotaxically using dental cement and bone screws to secure them to the skull. The cannulae were lowered in the sagittal plane following retraction of the superior sagittal sinus [22]. A 28-gauge stainless steel obturator which extended 0.5 mm beyond the end of the guide cannula was then inserted. Following surgery, sterile penicillin (1cc/kg, Durapen) was given to all rats. Subjects were allowed 7 days to recover from surgery.
Drugs
Muscimol (5-(aminomethyl)-isoxazol-3-ol, molecular weight (MW)=114.1), DAMGO (MW=513.6), DPDPE (MW=645.8), amphetamine (MW=135.2) and haloperidol (MW=375.9) were obtained from Sigma Chemical Company (St. Louis, MO) and pBB-PZDA (MW=357.2) and baclofen ((RS)-4-amino-3-(4-chlorophenyl)butanoic acid, MW=213.7) from Tocris Neuroamin (Essex, England). All compounds were prepared in sterile normal saline except for haloperidol which was prepared in 30% propylene glycol to which was added the minimum amount acetic acid necessary to dissolve the compound.
Intracranial drug injections
Microinjections were made through a 28-gauge stainless steel injection cannula connected to a motor driven Hamilton microsyringe by fluid filled polyethylene tubing. After the obturator was taken from the guide cannula, an injection cannula was inserted so that its tip extended 2mm beyond the end of the guide cannula. All injections were made in a volume of 0.5ul at a rate of 0.25ul/min. The injection cannula was removed 30sec after completion of the injection. The obturator was then replaced and the subject returned to the test chambers. All subjects were given one “sham injection,” in which no fluid was infused, prior to the start of behavioral testing in order to acclimate them to the procedure.
Locomotor activity measurement
Locomotor activity was measured in infrared photocell cages measuring 71.5 x 71.5 x 27cm. Four infra-red photobeams located 3.5 cm above the floor detected horizontal movements. The boxes were painted black and lighting provided by overhead fluorescent light fixtures. All subjects were given a one hour run in the activity boxes on day 7 following surgery to allow for adaptation to the test environment. On test days, rats received systemic (i.p.) injections of either saline or haloperidol, as detailed below, and were placed in the photocell cages for a 1 hr habituation period,. Animals were then were taken from the boxes and given either systemic or intracranial injections, after which they were returned to the test apparatus for a one hour period.
Experimental design
Rats were divided into 6 experimental groups containing 6–8 subjects each. One of these groups was unoperated and was used to study the effects of haloperidol on amphetamine induced hyperactivity. Subjects in the remaining five groups were prepared with intra-MR cannulae and were used to study the effects of haloperidol on the hyperactivity induced by intra-MR drug injections. Individual groups were tested with either the GABAA agonist muscimol (25ng), the GABAB agonist baclofen (62.5ng), the μ-opioid receptor agonist DAMGO (437ng), the δ-opioid agonist DPDPE (97.5ug) or the kainate/quisqualate antagonist pBB-PZDA (2.5ug). The doses were selected based on previous dose response studies conducted in our laboratory and were expected to produce roughly equivalent levels of hyperactivity. Each rat received four test runs in the activity boxes separated from each other by at least two intervening days. On one of these runs, subjects were injected systemically with saline (1 ml/kg, i.p.) followed, 60 min later, by intra-MR, or, in the case of the amphetamine group, systemic saline. On the remaining three runs, rats received injections of haloperidol (0.2 or 0.4 mg/kg, i.p), or its vehicle, immediately before placement in the activity boxes. One hour later the animals were briefly removed from the activity boxes and given intracranial injections of the drug to which their group had been assigned, or, in the case of the unoperated animals, of d-amphetamine sulphate (1.5 mg/kg, s.c). The order of the four test injections was randomized between animals in each group.
Cannula Placement confirmation
At the end of experiment, all operated rats were perfused transcardially, under deep pentobarbital anesthesia (100mg/kg), with normal saline followed by a 10% formalin solution. The brains were removed from the skulls and stored in fixative for at least 2 weeks after which 64 μm cryostat sections were cut through the sites of the injection cannulae and subsequently stained with cresyl violet to verify the injection locations.
Results
Histology
All cannula placements were located within the central MR at locations similar to those we have documented extensively in previous reports [6,17,23]. An example of a typical cannula placement is shown in Fig. 1.
Fig. 1.
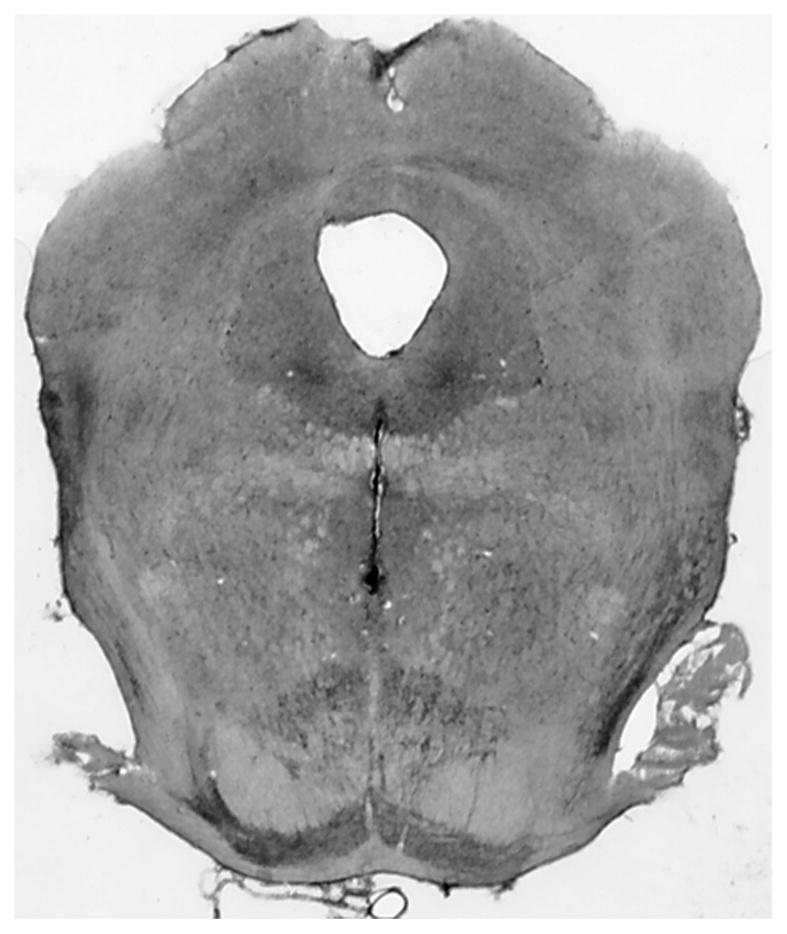
Example of a cresyl violet stained section through a typical cannula track terminating in the central portion of the MR.
Effects of haloperidol on baseline locomotor activity
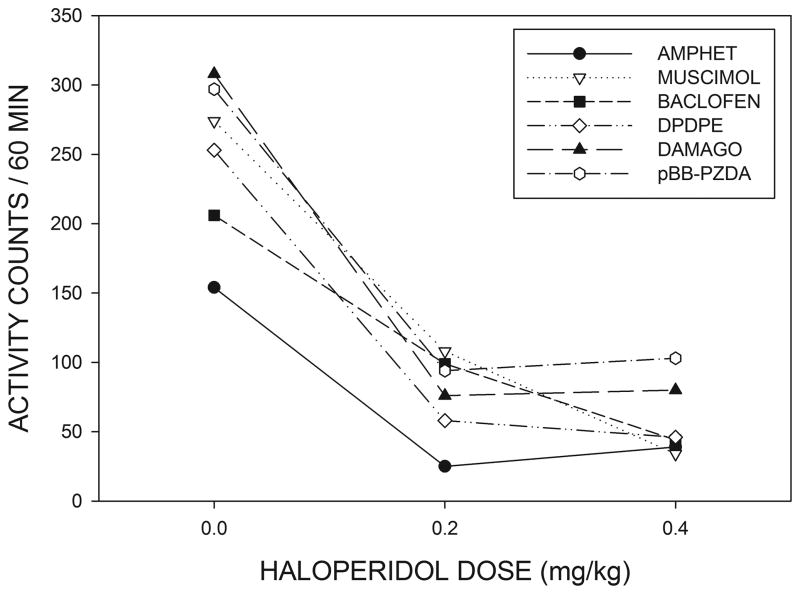
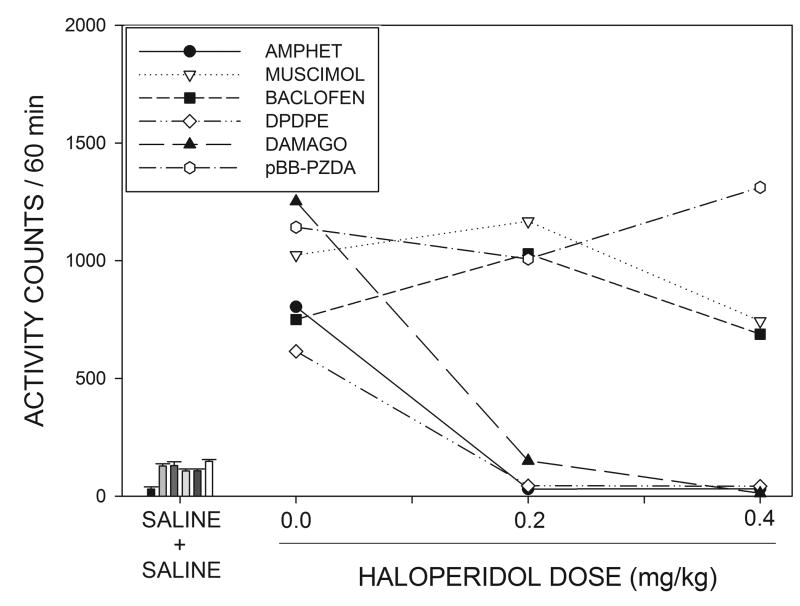
Fig 2 shows that haloperidol treatment produced a suppression of locomotor activity in rats with MR cannulae in the 60 min period preceding intra-MR drug injections. A 3 X 5 (haloperidol dose X intra-MR drug group) ANOVA indicated a significant effect of haloperidol treatment (F(2,54)=208.3, p<0.001) but differences between the different MR treatment groups were not significant (p>0.05). Haloperidol thus produced a similar reduction in spontaneous locomotion in all of the groups with MR cannulae. Fig. 2 also shows that haloperidol suppressed activity during the baseline period in the unoperated animals who were to later receive systemic amphetamine injections (F(2,10)=37.4, p<0.001). Baseline activity in the absence of haloperidol (i.e., after systemic saline injections) tended to be lower in these unoperated animals than in the groups of animals with intra-MR cannulae and a comparison between unoperated animals and the remaining subjects, collapsed across groups, was significant (F(1,36)=11.1, p<0.002).
Fig. 2.
Effects of systemic haloperidol on locomotor activity during the 60 min period prior to intracranial injections of the listed compounds or systemic injections of amphetamine. Haloperidol produced an equivalent reduction in locomotor activity in all of the drug groups (see text for details.)
Effects of haloperidol on locomotor activity induced by intra-MR drug injections
Locomotor activity in the 60 min period following intra-MR drug injections is shown in Fig. 3, where it can be seen that, in the absence of haloperidol, all of the intra-MR drug injections produced marked hyperactivity compared to locomotion following intra-MR saline injections, shown in the bar graphs on the left of the figure. An ANOVA indicated that the increase in locomotor activity over saline did not differ significantly between the 5 MR injection groups in the absence of haloperidol (p>0.1). Examination of Fig. 3 suggests that haloperidol was able to suppress the hyperactivity induced by intra-MR injections of the opioid agonists DPDPE and DAMGO, but had little apparent effect on the response to muscimol, baclofen or pBB-PZDA. These data were analyzed by means of a 3 X 5 (haloperidol dose X intra-MR drug group) ANOVA with repeated measures on the first factor. This analysis indicated a significant treatment X group interaction (F(8,54)=2.77, p<0.012) implying that haloperidol differentially affected the responses produced by different drug injections. In order to further clarify the nature of the interaction effect, individual one-way ANOVAs were conducted for each of the 5 intra-MR drug treatments across the three haloperidol dose levels. The Bonferroni correction was applied to the resulting p values. These results indicated that haloperidol significantly attenuated the responses to DPDPE (F(2,14)=36.4, p<.005,) and DAMGO (F(2,10)=9.63, p<0.025) but not those to muscimol, baclofen or pBB-PZDA (F<1 in all cases).
Fig. 3.
The line graphs on the right side of the figure show the effects of various doses of haloperidol on locomotor activity induced by systemic injections of amphetamine or intracranial injections of muscimol, baclofen, DPDPE, DAMGO or pBB-PZDA, See text for statistical details. The bar graphs on the left of the figure display locomotor activity under the control condition in which animals received systemic injections of saline followed by intracranial, or systemic, injections of saline. Bars are displayed in the same order as listed on the key, and subjects who did not receive cannula implants (i.e., those in the systemic amphetamine group) were less active than animals in the cannulated groups following saline injections.
Effects of haloperidol in rats treated with amphetamine
The bar graphs on the left of Fig. 3 show that the locomotion following systemic saline injections tended to be lower than that seen after intra-MR saline injections (F(1,36)=12.3, p<0.001, comparing saline to the pooled subjects in the remaining groups), but the locomotor activity produced by amphetamine was similar to that seen after the various intra-MR drug treatments. A one-way ANOVA indicated that the response to amphetamine was significantly attenuated by haloperidol pretreatment (F(2,10)=69.4, p<0.001).
DISCUSSION
The current experiments confirm that the MR exerts a major influence on locomotor activity; even the minor damage produced by cannula insertion appeared to be associated with a significant increase in locomotion relative to unoperated subjects. Much larger increases, however, could be produced by intra-MR injections of drugs acting at a number of different neurotransmitter receptors. The most important result of the current study is that the ability of the preferential D2 dopamine receptor antagonist haloperidol to suppress the response to these injections varied depending on the identity of the drug infused into the MR. Thus, haloperidol was able to abolish the hyperactivity induced by intra-MR injections of the opioid agonists DAMGO and DPDPE, but had little effect on the locomotor responses to the GABAA agonist muscimol, the GABAB agonist baclofen or the preferential non-NMDA glutamate antagonist pBB-PZDA. In contrast to its differential effects on drug induced activity in the various intra-MR treatment groups, haloperidol produced an equivalent reduction of locomotion in all of the cannulated groups during the baseline period before intra-MR injections. These findings indicate that the MR region must contain multiple mechanisms capable of influencing locomotor activity, some of which are dependent on dopamine, whereas others are not.
The findings of marked hyperactivity following injections of muscimol, baclofen and pBB-PZDA into the MR are in agreement with previous reports [6,8,9,24], and support the view that the MR exerts an inhibitory effect on behavioral arousal mechanisms. In the case of muscimol and baclofen, much larger effects on activity are seen when the drugs are injected into the MR than at adjacent sites such as the dorsal raphe nucleus (DR) or caudal VTA, suggesting these drugs are acting in the immediate vicinity of the MR [8,11]. Although mapping studies have not been conducted with pBB-PZDA, responses to a number of other glutamate antagonists have similarly been shown to be selective to the MR [6]. The concept that the MR plays a major role in the control of locomotor activity is consistent with reports that lesions of the MR produce much larger increases in locomotion than are seen after lesions of the adjacent DR [1–3]. A great deal of evidence suggests that the effects of both lesions and drug injections are not due primarily to alterations in serotonergic activity [2,9,10,13,25]. Although most workers have focused on the population of serotonin neurons found in the MR, these cells in fact make up a minority of the neurons in this area, and MR cells have been identified which synthesize a variety of other transmitters, including GABA and glutamate [12,26–28]. While the effects of muscimol and pBB-PZDA are likely to be mediated through an action on postsynaptic receptors, it is possible that the effects of baclofen are primarily presynaptic [29].
In addition to increasing locomotion, it has been shown that intra-MR muscimol, baclofen and glutamate antagonists are all able to increase dopamine metabolism in the nucleus accumbens [6,8,17,18]. Since dopamine receptor stimulation in this region is able to increase activity [19,20], a plausible hypothesis is that injections of these drugs into the MR produce hyperactivity as a result of increases in accumbens dopamine release. In the case of muscimol, however, previous studies have provided strong evidence against this theory by demonstrating that the locomotor response to this drug is unaffected by high doses of the D2 antagonist haloperidol [17]. The current study, confirms this finding by demonstrating that haloperidol, at doses which totally abolish amphetamine-induced hyperactivity, is without significant effect on the response to muscimol. Additionally, the current results show for the first time that the locomotor responses to both baclofen and pBB-PZDA are similarly independent of D2 receptor stimulation. It is striking that the behavioral effects of muscimol, baclofen and glutamate antagonists are very similar; for example, in addition to hyperactivity they all stimulate pronounced increases in food and water intake [23,30,31] and support self administration [32,33]. These findings further suggest that these drugs are acting on a common substrate. It is possible that the cell populations responsible for hyperactivity are either located downstream from dopaminergic mechanisms, or are components of a behavioral arousal system which is organized in parallel to dopaminergic pathways. In other studies (in preparation) we have found that stimulation of the lateral hypothalamus (LH) also gives rise to dopamine independent hyperactivity; this observation seems of special interest since the MR and LH are heavily interconnected and several lines of evidence suggest that some effects of MR manipulations may be mediated through the LH [30]. Even though D2 dopamine receptors may not play an essential role in the locomotor responses to certain drugs injected in the MR, it is possible that they may be involved in other actions of these drugs, such as their rewarding effects as assessed in self administration and conditioned place preference paradigms [32,33,55].
The ineffectiveness of haloperidol is especially striking because it indicates not only that the hyperactivity induced by these drugs does not result entirely from D2 receptor stimulation, but that these agents are actually able to overcome the akinesia otherwise induced by D2 receptor blockade. It is possible that inhibition of MR cells may in some way antagonize or disengage the mechanisms through which haloperidol suppresses locomotion. Clinical precedents exist for such a possibility; it has, for example, been suggested that parkinsonian motor signs disappear during REM sleep in patients with REM sleep behavior disorder, allowing these individuals to generate movements which would not be possible during normal waking [34]. It is also of interest that selective inhibition of serotonergic cells in the MR, a protocol which has only minor effects on locomotion, is able to antagonize haloperidol induced catalepsy [35]. It is possible that serotonin may be involved in reversing the akinetic effects of haloperidol, even if this transmitter does not play a major role in mediating the locomotor response to MR manipulations. Baclofen injections, however, do not appear to alter hippocampal serotonin release [8,36], even though GABAB receptors have been demonstrated on serotonin cells in the MR [29]. (Paradoxical effects of baclofen on serotonin release have also been observed in the DR, and have been suggested to result from presynaptic inhibition of GABA release [37,38]). These findings suggest that serotonin antagonism may not be essential for reversing haloperidol akinesia; it is possible, however, that baclofen affects serotonin release at sites other than the hippocampus, or that the effects of this drug on serotonin may have been difficult to detect with the methods employed in those studies.
The current findings raise the possibility that effects on dopamine turnover may be mediated through a different cell population than that involved in effects on locomotor activity. Substantial further work will be needed to evaluate this possibility and identify the neurons responsible for these two effects and to determine whether their anatomical distributions are identical.
The present results confirm reports of hyperactivity following intra-MR injection of μ-opioid agonists [7,39] but demonstrate that, in sharp contrast to the results obtained after injections of GABA agonists and EAA antagonists, the response to DAMGO is highly sensitive to haloperidol. Since DAMGO injections have been shown to increase dopamine metabolism within the accumbens [7], this finding suggests that the hyperactivity induced by this treatment may be secondary to alterations in dopamine release. These results indicate that the cell populations underlying the effects of DAMGO are not identical to those underlying the responses to GABA agonists and EAA antagonists. This possibility is supported by the fact that a number of differences are present between the effects of DAMGO and those of drugs acting on GABA or glutamate receptors. Intra-MR DAMGO, for example, does not stimulate food intake to nearly the extent as do GABAA or GABAB agonists or glutamate antagonists [7]. Unlike muscimol and glutamate antagonists, activation of MR μ-opiate receptors does not alter serotonin metabolism in either the hippocampus or the MR itself [7,39–41. Perhaps most importantly, equivalent increases in locomotor activity are seen when DAMGO is injected into either the MR or the far caudal VTA [7]. In contrast, GABAA and GABAB agonists and glutamate antagonists produce much larger responses when injected into the MR as opposed to the caudal portions of the VTA [6,8,11]. While it is possible that different cell populations may mediate the effects of DAMGO injected into these two regions, it is also possible that a single μ-opioid sensitive cell population may extend from the region of the MR into the vicinity of the caudal VTA and be affected by DAMGO injections at either site. An especially interesting possible candidate in this regard is the cell group which has been variously referred to as the juxtamedian cell group, the tail of the ventral tegmental area, or the rostromedial tegmental nucleus (RMTg), that lies on the lateral borders of the rostral MR and extends into the lateral portion of the caudal VTA [42–45]. This region contains GABAergic cells, is rich in opioid receptors, and has been shown to project to dopamine cells in the VTA and substantia nigra pars compacta [42,46]. Further studies will be needed to evaluate the involvement of this cell group in the response to μ-opioid agonists. However it should be noted both that many μ-opioid receptor expressing cells are found within the MR itself [47,48] and that the injection sites studied here were located well caudal to the bulk of RMTg cells.
The current data also demonstrate that blockade of D2 receptors is able to antagonize the hyperactivity induced by intra-MR injections of the δ-opioid agonist DPDPE [7]. Since intra-MR injections of this compound have been shown to increase dopamine metabolism in both the nucleus accumbens and the dorsal striatum [7], it is again plausible that these locomotor effects may be secondary to alterations in dopamine release. δ-opioid receptors have been shown to be present within the MR [49–51] and previous studies have demonstrated that DPDPE is much more effective at inducing hyperactivity when injected into the MR than the caudal VTA [7]. These results suggest that the hyperactivity induced by DPDPE is unlikely to be mediated through diffusion to the RMTg and thus indicate that dopamine dependent hyperactivity can be obtained from the MR itself. In previous studies we have found that DPDPE injections do produce a small decrease in hippocampal serotonin metabolism but, unlike drugs acting on GABA or glutamate transmission, appear unable to alter food or water intake [7]. Thus, the behavioral profile of DPDPE differs from that of the agents which produce dopamine-insensitive hyperactivity, further indicating that the substrate of DPDPE’s effects cannot be identical to that of GABAergic and glutamatergic agents.
In summary, the current results support two major conclusions. First, they strongly indicate that the MR region contains a substrate which is capable of exerting a powerful influence over behavioral arousal that is independent of D2 dopamine receptor stimulation. It is likely that the cell population that underlies this effect plays an important, and underappreciated, role in the control of behavioral activation. Since the MR region receives heavy afferent projections from the prefrontal cortex and a number of hypothalamic sites [52–54], it may play a role in mediating the influence of these structures on behavioral activation. Given the ability of certain agents in the MR to overcome effects of dopamine blockade, it is possible that pharmacological manipulation of this system might be of therapeutic relevance in parkinsonian syndromes. Second, our results, together with those of previous studies, demonstrate that different drugs injected into the MR produce different patterns of behavioral and neurochemical effects, suggesting that the paramedian tegmentum must contain multiple cell populations that differentially influence behavior and neural activity. Given the powerful and ubiquitous nature of the responses produced by manipulations of this region, it seems that a detailed characterization of the neural circuitry of the region is likely to substantially advance our understanding of the mechanisms underlying behavioral arousal.
Research Highlights.
We studied effects of haloperidol on median raphe nucleus (MR) induced-hyperactivity.
Glutamate antagonist pBB-PZDA in MR induced haloperidol insensitive hyperactivity.
GABA agonists muscimol and baclofen induced haloperidol insensitive hyperactivity.
Opioid agonists DAMGO and DPDPE induced haloperidol sensitive hyperactivity.
The effects of different drugs injected in MR are mediated through different pathways.
Acknowledgments
Supported in part by NIH grants RO3DA020802 and RO1DK071738. IS was supported by the 2013 research leave program grant from Kyung Hee University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Geyer MA, Puerto SA, Menkes DB, Segal DS, Mandell AJ. Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways. Brain Res. 2013;106:257–270. doi: 10.1016/0006-8993(76)91024-6. [DOI] [PubMed] [Google Scholar]
- 2.Asin KE, Fibiger HC. An analysis of neuronal elements within the median nucleus of the raphe that mediate lesion induced increases in locomotor activity. Brain Res. 1983;268:211–213. doi: 10.1016/0006-8993(83)90487-0. [DOI] [PubMed] [Google Scholar]
- 3.Asin KE, Wirtshafter D, Kent EW. Straight alley acquisition and extinction and open field activity following discrete electrolytic lesions of the mesencephalic raphe nuclei. Behav Neural Biol. 1979;25:242–256. doi: 10.1016/s0163-1047(79)90597-1. [DOI] [PubMed] [Google Scholar]
- 4.Lorens SA, Guldberg HC, Hole HC, Kohler C, Srebro B. Activity, avoidance lelarning and regional 5-hydroxytryptamine following intra-brain stem 5,7-dihydroxytryptamine and electrolytic midbrain raphe lesions in the reat. Brain Res. 1976;108:97–113. doi: 10.1016/0006-8993(76)90167-0. [DOI] [PubMed] [Google Scholar]
- 5.Wirtshafter D, Asin KE. Evidence that electrolytic median raphe lesions increase locomotion but not exploration. Physiol Behav. 1982;28:749–754. doi: 10.1016/0031-9384(82)90189-5. [DOI] [PubMed] [Google Scholar]
- 6.Wirtshafter D, Trifunovic R, Krebs JC. Behavioral and biochemical evidence for a functional role of excitatory amino acids in the median raphe nucleus. Brain Res. 1989;482:225–234. doi: 10.1016/0006-8993(89)91185-2. [DOI] [PubMed] [Google Scholar]
- 7.Klitenick MA, Wirtshafter D. Behavioral and neurochemical effects of opioids in the paramedian midbrain tegmentum including the median raphe nucleus and ventral tegmental area. J Pharmacol Exp Ther. 1995;273:327–336. [PubMed] [Google Scholar]
- 8.Wirtshafter D, Stratford TR, Pitzer MR. Studies on the behavioral activation produced by stimulation of GABA-B receptors in the median raphe nucleus. Behav Brain Res. 1993;59:83–93. doi: 10.1016/0166-4328(93)90154-i. [DOI] [PubMed] [Google Scholar]
- 9.Wirtshafter D, Klitenick MA, Asin KE. Evidence against serotonin involvement in the hyperactivity produced by injections of muscimol into the median raphe nucleus. Pharmacol Biochem Behav. 1987;27:45–52. doi: 10.1016/0091-3057(87)90475-8. [DOI] [PubMed] [Google Scholar]
- 10.Paris JM, Lorens SA. Intra-median raphe infusions of muscimol and the substance P analogue DiMe-C7 produce hyperactivity: role of serotonin neurons. Behav Brain Res. 1987;26:139–151. doi: 10.1016/0166-4328(87)90162-8. [DOI] [PubMed] [Google Scholar]
- 11.Wirtshafter D, Klitenick MA. Comparative studies of locomotor behavior following microinjections of muscimol into various sites in the paramedian tegmentum. Pharmacol Biochem Behav. 1990;32:625–628. doi: 10.1016/0091-3057(89)90008-7. [DOI] [PubMed] [Google Scholar]
- 12.Leger L, Wiklund L. Distribution and numbers of indoleamine cell bodies in the cat brainstem determined with the Flak-Hillarp fluorescence histochemistry. Brain Res Bull. 1981;9:245–251. doi: 10.1016/0361-9230(82)90137-x. [DOI] [PubMed] [Google Scholar]
- 13.Lorens SA. Some behavioral effects of serotonin depend on method: a comparison of 5,7-dihydroxytryptamine, p-chloroamphetamine and electrolytic raphe lesions. Ann N Y Acad Sci. 1978;305:532–535. doi: 10.1111/j.1749-6632.1978.tb31547.x. [DOI] [PubMed] [Google Scholar]
- 14.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic aferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 16.Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- 17.Wirtshafter D, Klitenick MA, Asin KE. Is dopamine involved in the hyperactivity produced by injections of muscimol into the median raphe nucleus. Pharmacol Biochem Behav. 1988;30:577–583. doi: 10.1016/0091-3057(88)90068-8. [DOI] [PubMed] [Google Scholar]
- 18.Wirtshafter D, Trifunovic R. Nonserotonergic control of nucleus accumbens dopamine metabolism by the median raphe nucleus. Pharmacol Biochem Behav. 1992;41:501–505. doi: 10.1016/0091-3057(92)90364-l. [DOI] [PubMed] [Google Scholar]
- 19.Pijnenburg AJ, Honig WM, van der Heyden JA, van Rossum JM. Effect of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976;35:45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- 20.Staton DM, Solomon PR. Microinjections of d-amphetamine into the nucleus accumbens and caudate-putamen differentially affect stereotypy and locomotion in the rat. Physiol Psychol. 1984;12:159–162. [Google Scholar]
- 21.Pellegrino LJ, Cushman AS. A Stereotaxic Atlas of the Rat Brain. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- 22.Wirtshafter D, Asin KE, Kent EW. Simple technique for midline stereotaxic surgery in the rat. Physiol Behav. 1979;23:409–410. doi: 10.1016/0031-9384(79)90388-3. [DOI] [PubMed] [Google Scholar]
- 23.Wirtshafter D, Stratford TR, Davis JD. Inactivation of the median raphe nucleus increases intake of sucrose solutions: A microstructural analysis. Behav Neurosci. 2011;125:529–540. doi: 10.1037/a0024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sainati S, Lorens SA. Intra-raphe muscimol-induced hyperactivity depends on ascending serotonin projections. Pharmacol Biochem Behav. 1982;17:973–986. doi: 10.1016/0091-3057(82)90482-8. [DOI] [PubMed] [Google Scholar]
- 25.Geyer MA, Peterson LR, Rose GJ. Effects of serotonergic lesions on investigatory responding by rats in a holeboard. Behav Neural Biol. 1980;30:160–177. doi: 10.1016/s0163-1047(80)91041-9. [DOI] [PubMed] [Google Scholar]
- 26.Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunocytochemistry. In: Brorklund A, Hokfelt T, editors. Handbook of Chemical Neuronatomy, Vol. 4, GABA and Neuropeptides in the CNS, Part 1. Amsterdam: Elsevier; 1985. [Google Scholar]
- 27.Aznar S, Knudsen GM. GABA containing neurons in dorsal and median raphe nucleus project to hippocampus and medial septumn/diagonal band of Broca comples. Soc Neurosci Abstr. 2002;2002:430.9. [Google Scholar]
- 28.Jackson J, Bland BH, Antle MC. Nonserotonergic projections neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse. 2009;63:31–41. doi: 10.1002/syn.20581. [DOI] [PubMed] [Google Scholar]
- 29.Wirtshafter D, Sheppard AC. Localization of GABA-B receptors in midbrain monoamine containing neurons in the rat. Brain Res Bull. 2001;56:1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- 30.Wirtshafter D. The control of ingestive behavior by the median raphe nucleus. Appetite. 2000;36:99–105. doi: 10.1006/appe.2000.0373. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher PJ. Effects of 8-OH-DPAT, 5-CT and muscimol on behavior maintained by a DRL20 schedule of reinforcement, following microinjection into the dorsal or median raphe nuclei. Behav Pharmacol. 1994;5:326–336. doi: 10.1097/00008877-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Shin R, Ikemoto S. The GABAB receptor agonist baclofen administered into the median and dorsal raphe nuclei is rewarding as shown by intracranial self-administration and conditioned place preference in rats. Psychopharmacol. 2010;208:545–554. doi: 10.1007/s00213-009-1757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb SM, Vollrath-Smith FR, Shin R, Jhou TC, Xu S, Ikemoto S. Rewarding and incentive motivational effects of excitatory amino acid receptor antagonists into the median raphe and adjacent regions of the rat. Psychopharmacol. 2012;224:401–412. doi: 10.1007/s00213-012-2759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnulf I. REM sleep behavior disorder: Motor manifestations and pathophysiology. 2012;6:689. doi: 10.1002/mds.24957. [DOI] [PubMed] [Google Scholar]
- 35.Inverrnizzi RW, Cervo L, Samanin R. 8-Hydroxy-2-(di-N-propylamine)tetralin, a selective serotonin-1A receptor agonist, blocks haloperidol-induced catalepsy by an action on raphe nuclei medianus and dorsalis. Neuropharmacol. 1988;27:515–518. doi: 10.1016/0028-3908(88)90134-7. [DOI] [PubMed] [Google Scholar]
- 36.Shim I, Javaid J, Wirtshafter D. Dissociation of hippocampal serotonin release and locomotor activity following pharmacological manipulations of the median raphe nucleus. Behav Brain Res. 1997;89:191–198. doi: 10.1016/s0166-4328(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 37.Abellan MT, Jolas T, Aghajanian GK, Artigas F. Dual control of dorsal raphe serotonergic neurons by GABAB receptors. Electrophysiological and microdialysis studies. Synapse. 2000;36:21–34. doi: 10.1002/(SICI)1098-2396(200004)36:1<21::AID-SYN3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Abellan MT, Adell A, Honrubia MA, Mengod G, Artias F. GABAB-R1 receptors in serotonergic neurons: effects of baclofen on 5-HT output in rat brain. NeuroReport. 2000;11:941–945. doi: 10.1097/00001756-200004070-00009. [DOI] [PubMed] [Google Scholar]
- 39.Tao R, Auerbach SB. Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neurosci. 1995;68:551–556. doi: 10.1016/0306-4522(95)00154-b. [DOI] [PubMed] [Google Scholar]
- 40.Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- 41.Spampinato U, Esposito E, Romandini S, Samanin R. Changes of serotonin and dopamine metabolism in various forebrain areas of rats injected with morphine either systemically or in the raphe nuclei dorsalis and medianus. Brain Res. 1985;328:89–95. doi: 10.1016/0006-8993(85)91326-5. [DOI] [PubMed] [Google Scholar]
- 42.Wirtshafter D, Stratford TR. Dopaminergic control of Fos expression in the midbrain tegmentum. Soc Neurosci Abstr. 2011;256:11. [Google Scholar]
- 43.Kaufling J, Waltisperger E, Bourdy R, Valera A, Veinante P, Freund-Mercer MJ, Barrot M. Pharmacological recruitment of the GABAergic tail of the ventral tegmental area by acute drug exposure. Brit J Pharmacol. 2010;161:1677–1691. doi: 10.1111/j.1476-5381.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirtshafter D. Fos-like immunoreactivity in basal ganglia outputs following administration of dopamine agonists. Paper presented at the annual meeting of the Society for Neuroscience; 1994. [Google Scholar]
- 46.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zham DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalyuzhny AE, Wessendorf MW. CNS GABA neurons express the μ-opioid receptor: immunocytochemical studies. NeuroReport. 1997;8:3367–3372. doi: 10.1097/00001756-199710200-00035. [DOI] [PubMed] [Google Scholar]
- 48.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 49.Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- 50.Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Low HH, Elde R, Wessendorf MW. δ-Opioid receptor immunoreactivity: Distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvira-Botero MX, Garzon M. Cellular and subcellulalr distributions of delta opioid receptor activation sites in the ventral oral pontine tegmentum of the cat. Brain Res. 2006;1123:101–111. doi: 10.1016/j.brainres.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 52.Aghajanian GK, Wang RY. Habenular and other midbrain raphe afferents demonstrated by a modified retrograde tracing technique. Brain Res. 1977;122:229–242. doi: 10.1016/0006-8993(77)90291-8. [DOI] [PubMed] [Google Scholar]
- 53.Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neurosci. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- 54.Saper CB, Swanson LW, Cowan WM. An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J Comp Neurol. 1979;183:689–706. doi: 10.1002/cne.901830402. [DOI] [PubMed] [Google Scholar]
- 55.Liu ZH, Ikemoto S. The midbrain raphe nuclei mediate primary reinforcement via GABA(A) receptors. Eur J Neurosci. 2007;25:735–743. doi: 10.1111/j.1460-9568.2007.05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]