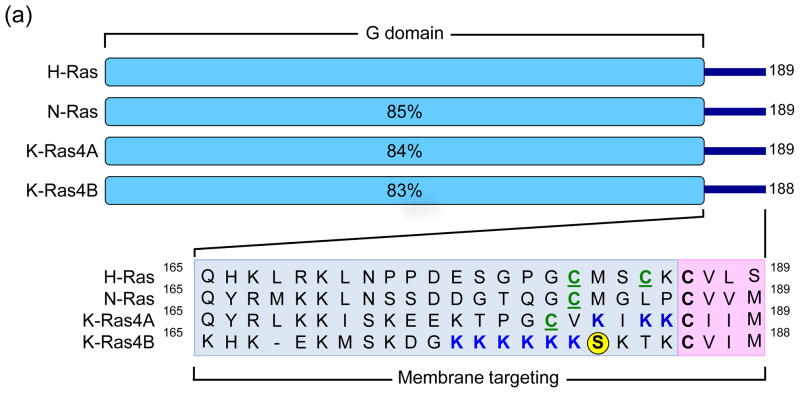
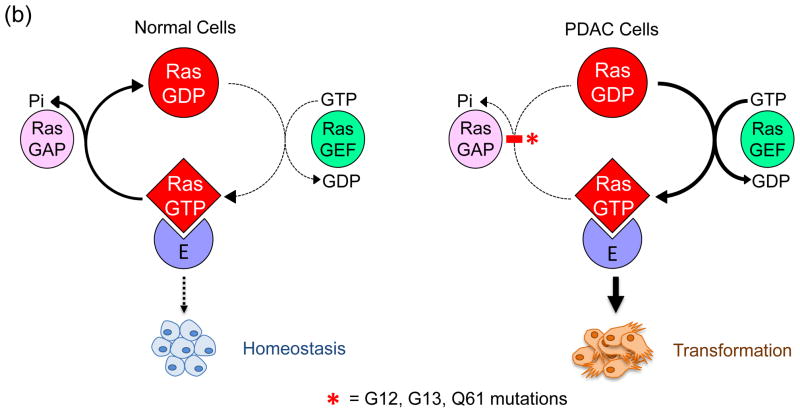
Figure 1.
Human Ras proteins are small GTPases. (a) The human RAS genes encode 188 or 189 amino acid proteins that share strong (82–90% overall) amino acid identity; percentages indicate identity with H-Ras. KRAS encodes two related proteins (K-Ras4A or K-Ras4B; 90% identity) due to alternative exon four utilization, with KRAS4B the predominant transcript in pancreatic tissue. Residues 1–164 comprise the G domain that binds and hydrolyses GTP (93–99% sequence identity). The remaining 24/25 C-terminal residues (shown in inset) comprise the membrane targeting sequence (16–40% identity), where the C-terminal four residues comprise the CAAX motif (shaded in pink; C = cysteine, A = aliphatic; X = terminal amino acid) that signals for farnesyltransferase-catalyzed covalent addition of a C15 farnesyl group to the cysteine residue. The 20/21 amino acids upstream of the CAAX motif comprise the hypervariable (HV) region (shaded in blue) where the Ras proteins exhibit the greatest sequence divergence. Within the HV domain are additional membrane targeting sequence elements that include cysteines that are covalently modified by addition of a palmitate fatty acid (green, underlined ‘C’) or polybasic sequences that promote association with the membrane (blue K). K-Ras4B contains a serine (S181) that is reversibly phosphorylated (yellow circle), regulating localization between the plasma and endomembranes. (b) Mutant K-Ras is persistently GTP-bound and active. Wild type K-Ras cycles between an active GTP-bound and an inactive GDP-bound state. In normal quiescent cells, K-Ras is predominantly GDP-bound. Upon growth factor stimulation, RasGEF activation promotes transient formation of K-Ras-GTP, promoting its binding to downstream effectors (E; e.g., Raf, PI3K). The cycle is terminated by the action of RasGAPs, returning K-Ras to the inactive GDP-bound state. Single amino acid substitutions at G12, G13 or Q61 impair the intrinsic GTP binding and hydrolytic activity of K-Ras, and additionally render the protein insensitive to GAP stimulation, favoring accumulation of persistently GTP-bound and active K-Ras in PDAC. Arrow line thickness corresponds to the level of signaling, i.e. in normal cells Ras is predominantly in the GDP-bound state due to relatively higher GAP activity and thus signals less to downstream effectors.