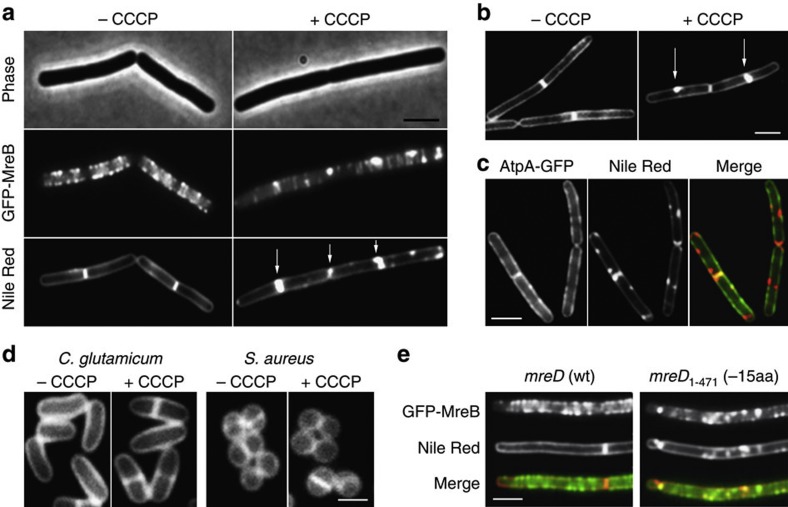
Figure 1. Disruption of the MreB cytoskeleton is accompanied by an aberrant membrane stain.
(a) Phase contrast images of B. subtilis cells (upper panel) expressing GFP-MreB (middle panel), and fluorescent Nile Red membrane stains (lower panel), are depicted in the absence or presence of the proton ionophore CCCP. Some of the Nile Red foci appearing with CCCP are highlighted with arrows (see Supplementary Fig. 5a for more examples). The CCCP-triggered local enrichment of MreB is not caused by the weak dimerization property of GFP, which was recently shown to stimulate protein clustering69 (Supplementary Fig. 5b), or by artificial overproduction (Supplementary Fig. 6). Strain used: B. subtilis YK405 (gfp-mreB). (b) Incubation of F1Fo ATP synthase-deficient cells with CCCP also results in a rapid (within 2 min) emergence of Nile Red foci, ruling out that the appearance of Nile Red foci is due to a reduction of ATP levels (see Supplementary Fig. 1c for more examples). Strain used: B. subtilis HS13 (Δatp). (c) Comparison of Nile Red foci, and the localization of the integral membrane protein F1Fo ATP synthase fused to GFP (AtpA-GFP). The lack of overlap in fluorescence signals rules out membrane invagination, or aberrantly formed septa (see arrows) as an explanation for the strong local Nile Red fluorescence (see Supplementary Fig. 4 for more examples). The reason that in some cases the Nile red stain appears to protrude into the cytoplasm is a consequence of high membrane fluorescence originating from slightly above and below the exact focal plane. Strain used: B. subtilis BS23 (atpA-gfp). (d) CCCP does not affect Nile Red fluorescent membrane stain in Staphylococcus aureus and Corynebacterium glutamicum which both lack MreB (see Supplementary Fig. 1d for more examples). Strains used: C. glutamicum RES167 and S. aureus RN4220. (e) Colocalization of GFP-MreB (upper panel) with Nile Red (middle panel) in B. subtilis strains encoding wild type MreD (wt), and a 15 amino acid C-terminal truncation of MreD (−15aa), which results in a mild delocalization of MreB in low Mg2+ medium (see Supplementary Fig. 1e for more examples). Strains used B. subtilis HS35 (gfp-mreB mreD wt) and HS38 (gfp-mreB mreD1-471/−15aa). Scale bar, 2 μm.