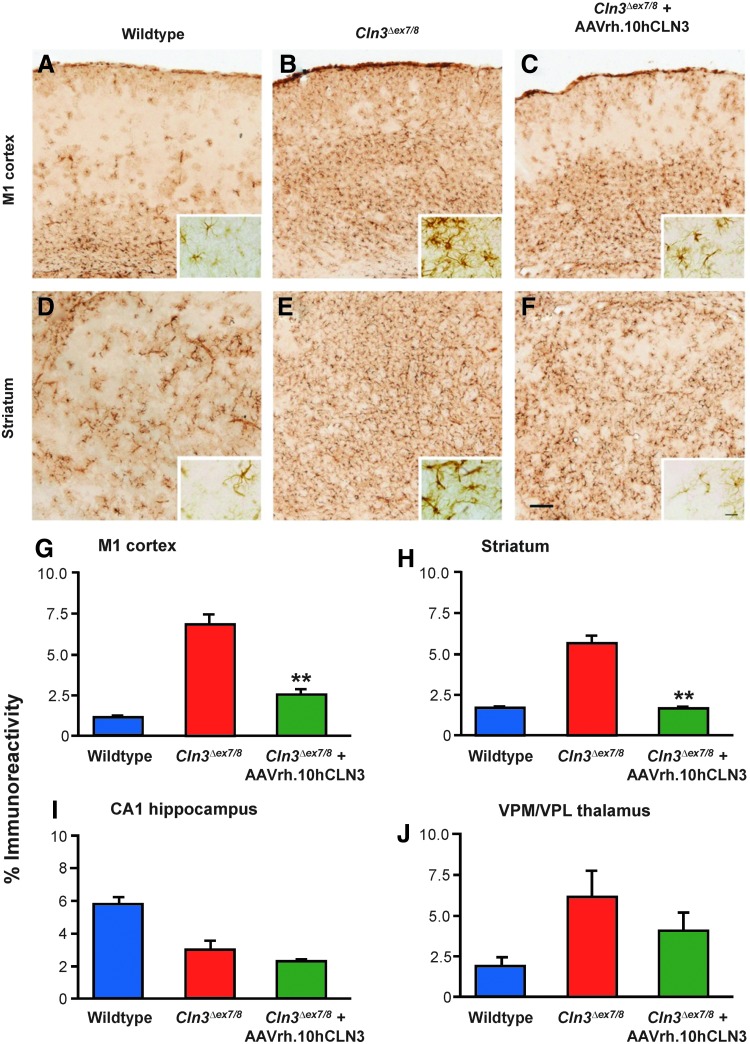
FIG. 7.
Effects of AAVrh.10hCLN3 administration upon astrocytosis. (A–F) Immunostaining for GFAP reveals the extent of astrocytosis in the M1 cortex (A–C) and striatum (D–F) of wild-type mice (A, D), untreated Cln3Δex7/8 mice (B, E), and AAVrh.10hCLN3-treated Cln3Δex7/8 mice (C, F). Compared with the scattered, faintly stained, and mostly protoplasmic astrocytes present in wild-type mice (A, D), widespread astrocytosis with more intensely stained astrocytes with thickened processes and hypertrophied cell soma were present throughout both regions of untreated Cln3Δex7/8 mice (B, E). In contrast, AAVrh.10hCLN3-treated Cln3Δex7/8 mice displayed less widespread astrocytosis, with more pronounced effects within the more dorsal cortical laminae (C), and in localized patches within the striatum (F). Scale bar=200 μm (20 μm in inserts). (G–J) Histograms of mean area of immunoreactivity per field (%) obtained by thresholding image analysis of GFAP immunostaining in the M1 cortex (G), striatum (H), CA1 subfield of the hippocampus (I), and ventral posterior (VPM/VPL) nucleus of the thalamus (J). These data confirm the disease-associated astrocytosis in these brain regions, as previously described (Pontikis et al., 2005), being elevated in all regions except hippocampal CA1. AAVrh.10hCLN3 administration decreased the level of GFAP immunoreactivity in all regions, but this reached statistical significance only in the M1 cortex and striatum. **p<0.01, one-way ANOVA with Bonferroni correction.