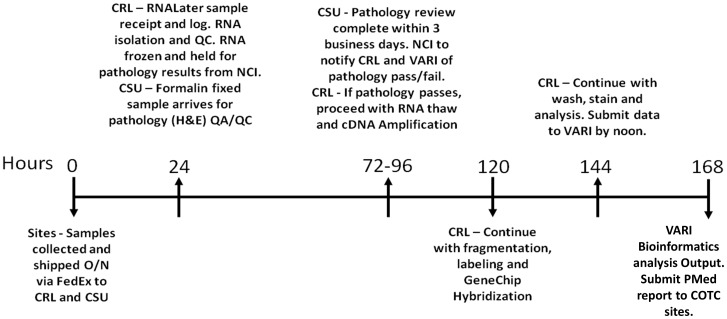
Figure 3. Expected clinical turnaround time for canine tumor sample collection, processing, expression, bioinformatic analysis and PMed report delivery.
The graphic defines the prospective timeline of key steps in the process of sample collection, shipment, histopathology and RNA quality assurance and control assessments, expression profiling and PMed report generation. Samples were biopsied at their clinical COTC site, sent to histopathology and CLIA labs for parallel sample and RNA QA/QC, Affymetrix gene expression analysis performed, and the derived genomic data sent to the Van Andel Research Institute for bioinformatics evaluation and PMed report generation. Minimum feasible turnaround time for sample analysis was described prospectively as 7 business days (168 hours), however all cases were completed in 4.85 days (116.46 hours). The process was successful in defining high quality tissues for molecular analysis and will be used in future canine PMed comparative studies.