Abstract
Background:
Several case reports have suggested that women’s use of exogenous sex hormones is associated with acute pancreatitis; however, relevant epidemiologic data are sparse. We examined the association between postmenopausal hormone replacement therapy and risk of acute pancreatitis.
Methods:
We conducted a prospective study involving 31 494 postmenopausal women (aged 48–83 yr) from the population-based Swedish Mammography Cohort. Participants completed a baseline questionnaire in 1997 assessing their use of hormone replacement therapy. We linked the cohort to the hospital-based Swedish National Patient Register to determine hospital admissions for acute pancreatitis through 2010. Relative risks (RRs) were calculated using Cox proportional hazard models.
Results:
Over a total follow-up of 389 456 person-years, we identified 237 cases of incident acute pancreatitis. The age-standardized incidence rates per 100 000 person-years were 71 cases among women who had ever used hormone replacement therapy and 52 cases among women who had never used such hormones. Among ever users of hormone replacement therapy, the multivariable-adjusted RR of acute pancreatitis was 1.57 (95% confidence interval [CI] 1.20–2.05) compared with never users. The risk did not differ by current or past use, but it seemed to be higher among women who used systemic therapy (RR 1.92, 95% CI 1.38–2.66) and among those with duration of therapy of more than 10 years (RR 1.87, 95% CI 1.11–3.17).
Interpretation:
Use of postmenopausal hormone replacement therapy was associated with increased risk of acute pancreatitis. Physicians should consider this potential increase in risk when prescribing such therapy.
The clinical spectrum of acute pancreatitis varies from a mild, self-limiting illness to a severe or fatal disease.1 Besides well-established risk factors such as alcohol abuse and gallstone disease,2 the use of certain prescription drugs has been suspected as a cause of acute pancreatitis (as reviewed by Trivedi and Pitchumoni3 and by Nitsche and associates4). Hormone replacement therapy, which is widely used to relieve postmenopausal symptoms, may, according to several case reports,5–10 increase the risk of acute pancreatitis. Such an association has also been suggested for oral contraceptives.11–14 However, epidemiologic data on the association between use of exogenous sex hormones and risk of acute pancreatitis are sparse. One case–control study from Denmark showed no clear increase in risk with short-term (≤ 1 yr) use of postmenopausal hormone replacement therapy.15 Whether a longer duration of therapy increases the risk of acute pancreatitis has not been studied.
We sought to evaluate prospectively the association between hormone replacement therapy and risk of acute pancreatitis in a cohort of postmenopausal women.
Methods
Participants
The Swedish Mammography Cohort is a population-based prospective study conducted in central Sweden. From 1987 to 1990, all women born between 1914 and 1948 and living in Västmanland and Uppsala counties (n = 90 303) were invited to participate in a mammography screening program and to complete a dietary questionnaire. Of these, 66 651 women (74%) returned a completed questionnaire. In 1997, a second questionnaire was sent to all surviving women (n = 56 030), with questions on such lifestyle factors as diet, alcohol intake and smoking status, as well as questions on medications and medical history; here, the response rate was 70% (n = 39 227).
For the current study, only those who completed the second questionnaire (in 1997) were eligible, given that hormone replacement therapy was not mentioned in the first questionnaire. Of these, we excluded women with an incorrect personal identity number, those with a history of cancer (except nonmelanoma skin cancer) or exocrine pancreatic disease, and those who, according to the questionnaire, were premenopausal (as previously defined16) or had missing information on hormone replacement therapy.
Ethics approval was granted by the Regional Ethical Review Board at Karolinska Institutet (Stockholm, Sweden), and return of a completed questionnaire was considered to imply informed consent.
Assessment of hormone replacement therapy
The questionnaire asked participants to report their use of hormone replacement therapy, whether such therapy was currently in progress, the duration of use and the indication for use (hot flashes or vaginal dryness, referred to hereafter as “systemic therapy” and “local therapy,” respectively). Self-reported use of hormone replacement therapy has been shown to be highly comparable with prescription data (kappa = 0.90).17
Assessment of covariables
From the questionnaire, we obtained information on age at menopause, age at menarche, use of oral contraceptives, parity, education, smoking status, body mass index (BMI), waist circumference, alcohol intake and vegetable consumption. We obtained baseline history of cholelithiasis and diabetes mellitus from the Swedish National Patient Register and the Swedish National Diabetes Register, respectively. Additional information on diabetes was acquired from the questionnaire.
Outcome measure
We identified cases of incident acute pancreatitis (International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10] code K85) between Sept. 15, 1997, and Dec. 31, 2010, by linkage to the Swedish National Patient Register. We acquired information on other exocrine pancreatic diseases (ICD-10 codes K86–K87), cholelithiasis (ICD-10 code K80) and gallbladder surgery (Nordic Medico-Statistical Committee Classification of Surgical Procedures codes JKA20, JKA21, JKE00, JKE02, JKE12, JKE18 or JKB30) from the same register. Finally, we obtained information on pancreatic cancer (ICD-10 code C25) and death from the Swedish National Cancer Registry and the Swedish National Cause of Death Register, respectively.
The Swedish National Patient Register covers nearly all hospital admissions in Sweden18 and was recently shown to have a positive predictive value of 98% (83% with strict criteria) for a recorded diagnosis of acute pancreatitis when validated by chart review.19 The register does not cover patients’ contacts or visits in primary health care.
Statistical analysis
We calculated person-years from Sept. 15, 1997, to the date of either acute pancreatitis, other exocrine pancreatic diseases (including cancer), death or Dec. 31, 2010, whichever occurred first.
We used Cox proportional hazard models, with age as the time scale,20 to estimate hazard ratios (referred to hereafter as relative risk [RR]) for acute pancreatitis associated with use of hormone replacement therapy. We classified participants as never users or ever users of hormone replacement therapy. We further classified ever users according to current use (no or yes), duration of use (< 5, 5–10 or > 10 yr) and type of therapy (local or systemic). In the analysis of type of therapy, we excluded women who reported having ever used both types of therapy. Furthermore, we modelled the association between duration of use and risk of acute pancreatitis using restricted cubic splines with knots at 10%, 50% and 90% of the distribution among ever users.21 We obtained a p value against the hypothesis of a linear association by testing the coefficient of the second spline transformation equal to 0. We tested the proportional hazards assumption (by scaled Schoenfeld residuals22) and observed no significant deviations.
Multivariable analyses were adjusted for age at menopause (< 51 or ≥ 51 yr; median 51 yr), education (primary school, high school or university), smoking status (never, past [as < 10 or ≥ 10 pack-years] or current [as < 20 or ≥ 20 pack-years]), level of adiposity (BMI < 25, 25–29 or ≥ 30 or waist circumference < 80, 80–87 or ≥ 88 cm, depending on the model), alcohol intake (average [quartiles of grams per day] or per occasion [< 1, 1, 2–3 or ≥ 4 drinks, where 1 drink = 12 g of alcohol], depending on the model) and vegetable consumption (quartiles of servings per day). For each covariable, if necessary, we included an indicator level for missing data. We performed sensitivity analyses on complete case data and on multiple imputed data (30 datasets were imputed using chained equations).23
We considered gallstone disease to be a mediator of the association of interest; thus, we adjusted for baseline history of cholelithiasis (no or yes) only in a sensitivity analysis. We also performed analyses using non–gallstone-related acute pancreatitis as the outcome (defined as all K85 codes, except K85.1, without concurrent cholelithiasis or gallbladder surgery ≤ 3 months after the index episode24).
We performed several sensitivity analyses to account for changes in use of hormone replacement therapy during the follow-up period. First, we censored the analysis after 2003 because of the expected decrease in use following publication of findings from the Women’s Health Initiative.25 Second, we restricted the analysis to women older than 60 years, because women not taking hormone replacement therapy by that age would be unlikely to initiate such treatment later. Finally, we restricted the analysis of duration of use to past users, because their reported duration of use was unlikely to have changed.
We performed all analyses with Stata 12.1 (StataCorp, Tex.). Statistical tests were 2-sided with a 0.05 significance level.
Results
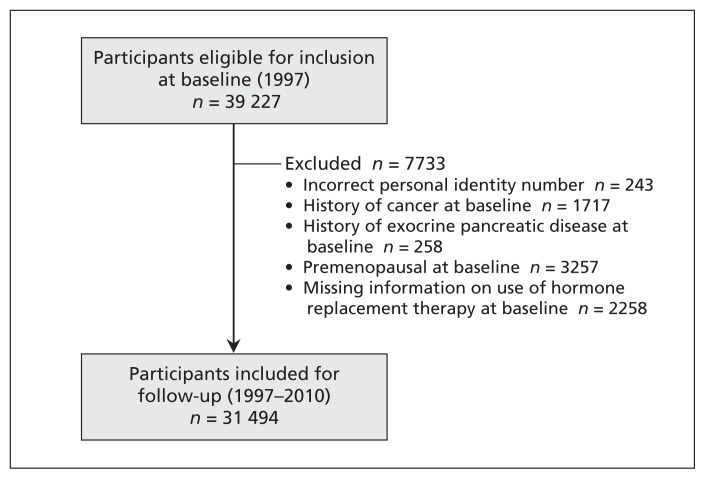
Of the 39 227 women who completed the questionnaire in 1997, a total of 31 494 postmenopausal women were included in the analysis (Figure 1). During the follow-up period (1997–2010; 389 456 person-years), we identified 237 cases of incident acute pancreatitis. The period prevalence of cholelithiasis was higher among those in whom acute pancreatitis occurred than among those without the disease (133/237 [56%] v. 4142/31 257 [13%]).
Figure 1:
Flow chart for participants in the Swedish Mammography Cohort whose data were used in an analysis of acute pancreatitis in relation to postmenopausal hormone replacement therapy.
At baseline, 13 113 (42%) of the women were current users of hormone replacement therapy, and 3660 (12%) were past users. Among current users, 6795 (52%) used systemic therapy, 4148 (32%) used local therapy and 2170 (17%) used both therapies. Past and current users were more likely to be well educated than never users (Table 1). In addition, compared with never users and past users, current users were younger, had lower BMI, were less likely to be current smokers and tended to have a higher alcohol intake. Past users were more likely to have a history of cholelithiasis.
Table 1:
Characteristics of 31 494 postmenopausal women in the Swedish Mammography Cohort, by use of HRT at baseline (1997)
| Characteristic*† | Use of HRT | ||
|---|---|---|---|
| Never | Past | Current | |
| No. of women | 14 721 | 3 660 | 13 113 |
| Age, yr, mean ± SD | 64 ± 9 | 64 ± 9 | 60 ± 8 |
| Age at menopause ≥ 51 yr, % | 48 | 47 | 46 |
| University education, % | 14 | 18 | 22 |
| Current smoker, % | 21 | 21 | 17 |
| BMI ≥ 30, % | 12 | 12 | 9 |
| Alcohol intake, g/d, mean | 5 | 5 | 6 |
| Vegetable consumption, servings/d, mean | 3 | 3 | 3 |
| History of cholelithiasis, % | 9 | 11 | 9 |
Note: BMI = body mass index, HRT = hormone replacement therapy, SD = standard deviation.
Values (except age) are standardized to the age distribution (≤ 51, 52–56, 57–61, 62–66, 67–71, 72–76, and ≥ 77 yr) of the study population.
Means and percentages were calculated for women with known values. Data were missing on age at menopause for 4 415 women, on education for 193, on BMI for 591, on smoking for 611, on alcohol intake for 1219 and on vegetable consumption for 237.
We observed a positive association between use of hormone replacement therapy and risk of acute pancreatitis (Table 2). Incidence rates, directly standardized to the cohort’s age distribution, were 71 cases per 100 000 person-years among women who had ever used hormone replacement therapy and 52 cases per 100 000 person-years among women who had never used such hormones. Ever users had a multivariable-adjusted RR of acute pancreatitis of 1.57 (95% confidence interval [CI] 1.20–2.05) compared with never users. The overall fraction of missing data was 21% in the multivariable model. Results based on complete case data (RR 1.64, 95% CI 1.22–2.20) and multiple imputed data (RR 1.51, 95% CI 1.16–1.97) were similar. Controlling for waist circumference26 and amount of alcohol consumed per occasion,27 rather than BMI and average alcohol intake, did not materially change the results (RR 1.53, 95% CI 1.17–2.01), nor were the results altered by further adjustment for age at menarche (< 13, 13 or > 13 yr), parity (0, 1–2 or > 2), oral contraceptive use (never or ever) and history of diabetes (no or yes) (RR 1.58, 95% CI 1.21–2.08). The risk of acute pancreatitis did not differ by current or past use of hormone replacement therapy but seemed to be higher among women who used systemic therapy (RR 1.92, 95% CI 1.38–2.66) and among those with duration of therapy of more than 10 years (RR 1.87, 95% CI 1.11–3.17) (Table 2). Among women who had used systemic therapy for more than 10 years (n = 945, including 14 cases of acute pancreatitis), the RR was 2.44 (95% CI 1.38–4.31). The restricted cubic spline analysis yielded no strong evidence against the hypothesis of a linear association between duration of hormone replacement therapy and risk of acute pancreatitis (p = 0.37). The RR for each 2 years of added use was 1.09 (95% CI 1.04–1.14).
Table 2:
Risk of acute pancreatitis associated with use of postmenopausal HRT (1997–2010)
| HRT | No. of women | No. of cases/person-years | Age-adjusted RR (95% CI) | Multivariable RR (95% CI)* |
|---|---|---|---|---|
| Never users | 14 721 | 98/178 430 | 1.00 (ref) | 1.00 (ref) |
| Ever users | 16 773 | 139/211 026 | 1.41 (1.08–1.83) | 1.57 (1.20–2.05) |
| Current use | ||||
| No | 3 660 | 36/45 144 | 1.49 (1.01–2.18) | 1.58 (1.07–2.32) |
| Yes | 13 113 | 103/165 882 | 1.38 (1.04–1.83) | 1.56 (1.17–2.09) |
| Duration of use, yr† | ||||
| < 5 | 5 937 | 46/75 238 | 1.45 (1.01–2.07) | 1.59 (1.10–2.29) |
| 5–10 | 3 463 | 32/43 797 | 1.53 (1.02–2.29) | 1.73 (1.14–2.61) |
| >10 | 1 372 | 17/16 648 | 1.62 (0.97–2.71) | 1.87 (1.11–3.17) |
| Type of therapy used‡ | ||||
| Local | 4 307 | 38/53 099 | 1.21 (0.83–1.76) | 1.37 (0.94–2.00) |
| Systemic | 9 115 | 75/116 419 | 1.66 (1.21–2.28) | 1.92 (1.38–2.66) |
Note: CI = confidence interval, HRT = hormone replacement therapy, ref = reference group, RR = relative risk.
Derived from a Cox proportional hazards model with age as time scale and with adjustment for age at menopause (< 51 or ≥ 51 yr), education (less than high school, high school or university), smoking status (never, past [as < 10 or ≥ 10 pack-years] or current [as < 20 or ≥ 20 pack-years]), body mass index (< 25, 25–29 or ≥ 30), alcohol intake (quartiles [g/d]) and vegetable consumption (quartiles [servings/d]).
Sum of categories is less than the total of 16 773 women who were ever users because data on duration of use were missing for 6001 women (including 44 cases).
Categories refer to women who were exposed exclusively to either systemic therapy or local therapy. In addition, 3351 women (including 26 cases) reported having ever used both types of therapy.
Adjustment for baseline history of cholelithiasis did not change the association between ever having used hormone replacement therapy and risk of acute pancreatitis (RR 1.57, 95% CI 1.20–2.06). The association was also maintained when we used non–gallstone-related acute pancreatitis as the outcome: based on 43 cases in never users and 72 cases in ever users, the RR for ever having used hormone replacement therapy was 1.96 (95% CI 1.32–2.90).
Overall, the association with ever having used hormone replacement therapy did not change appreciably in the sensitivity analyses (where follow-up was censored or additional restrictions were applied) (Table 3). Also, as in the main analysis, the risk of acute pancreatitis seemed to increase with duration of use.
Table 3:
Sensitivity analyses for risk of acute pancreatitis associated with use of postmenopausal HRT
| Sensitivity analysis | No. of women | No. of cases/person-years | Multivariable RR (95% CI)* |
|---|---|---|---|
| Follow-up until end of 2003 | |||
| Never user | 14 721 | 43/89 916 | 1.00 (ref) |
| Ever user | 16 773 | 57/103 756 | 1.52 (1.00–2.29) |
| Women aged > 60 yr | |||
| Never user | 9 080 | 76/105 441 | 1.00 (ref) |
| Ever user | 7 362 | 78/88 606 | 1.42 (1.03–1.97) |
| Women not currently using HRT† | |||
| Never user | 14 721 | 98/178 430 | 1.00 (ref) |
| Past user with duration < 5 y | 2 124 | 14/26 440 | 1.16 (0.66–2.04) |
| Past user with duration ≥ 5 y | 918 | 10/11 305 | 1.62 (0.84–3.13) |
Note: CI = confidence interval, HRT = hormone replacement therapy, ref = reference group, RR = relative risk.
Adjusted for the same covariables as the multivariable model (Table 2): age at menopause (< 51 or ≥ 51 yr), education (less than high school, high school or university), smoking status (never, past [as < 10 or ≥ 10 pack-years] or current [as < 20 or ≥ 20 pack-years]), body mass index (< 25, 25–29 or ≥ 30), alcohol intake (quartiles [g/d]) and vegetable consumption (quartiles [servings/d]).
Sum of categories is less than the total number of women not currently using HRT because data on duration of use were missing for 618 women (including 12 cases).
Interpretation
In this prospective cohort study, use of postmenopausal hormone replacement therapy was associated with increased risk of acute pancreatitis. Physicians should consider this potential increase in risk when prescribing this type of therapy.
Except for case reports,5–10 the epidemiologic data on the association between postmenopausal hormone replacement therapy and acute pancreatitis are sparse. Only 1 case–control study (n = 1054 cases of acute pancreatitis),15 which used data from prescription databases and hospital registers in Denmark, has examined the risk of acute pancreatitis associated with current and past use of hormone replacement therapy (defined as a prescription for such therapy within 1–90 or 91–365 days, respectively). That study showed a borderline significant association with past, but not current, use of combined estrogen and progestin preparations (odds ratio 1.6, 95% CI 1.0–2.5). No association was observed with estrogen-only preparations. Although there was adjustment for the occurrence of diseases related to alcohol and gallstones, the study did not adjust for other potential confounders, such as smoking28 and adiposity.26 Moreover, it did not consider an association with a longer duration of hormone replacement therapy, which limits the comparison with our study.
Because hormone replacement therapy may increase the risk of gallbladder disease,29 the observed association between hormone replacement therapy and acute pancreatitis could, in principle, be mediated by gallstones. However, the association remained when we adjusted for cholelithiasis, as well as when we used non–gallstone-related acute pancreatitis as the outcome, which suggests that hormone replacement therapy might have additional effects that may explain our findings. Most case reports on estrogen-induced pancreatitis have reported concurrent hypertriglyceridemia.7–12,14 Use of hormone replacement therapy may elevate triglycerides,30 which, even at moderately elevated levels, have been associated with increased risk of acute pancreatitis.31 The role of triglycerides in the pathogenesis of acute pancreatitis is not fully understood, but a key event is thought to be accumulation of free fatty acids in the pancreas after hydrolysis of triglycerides by pancreatic lipase.31 Exogenous estrogen may also have deleterious effects on the pancreas by itself, as some case reports have reported estrogen-induced pancreatitis in the absence of gallstones and hypertriglyceridemia.5,6,13 Experimental studies have identified estrogen-binding proteins in the pancreas,32 and upregulation of these proteins has been observed in the serum of patients with acute pancreatitis.33 Moreover, administration of high-dose estrogen has been reported to decrease the cholecystokinin-stimulated secretion of amylase in rats.34 Impaired secretion of pancreatic enzymes is considered an important event in the initial pathogenesis of acute pancreatitis.35
In other population-based Swedish studies based on data from the mid-1990s, oral estradiol combined with testosterone-like progestins was reported to be predominantly used as systemic therapy,36,37 whereas oral estriol and vaginal estriol, dienoestrol or estradiol were reported to be equally used as local therapy. Our finding that local therapy was associated with lower increase in risk of acute pancreatitis may therefore be due to local administration of estrogens of low dose or low potency. There are no contemporary data that might explain our finding that the risk was sustained among past users of hormone replacement therapy or that the risk seemed to increase with duration of use. These findings, though speculative, may suggest that exogenous estrogen induces some persistent change in the pancreas for which the duration of exposure may be important.
The strengths of our study were its prospective design, high response rate, virtually complete follow-up and adjustment for several potential confounders. The incidence of acute pancreatitis was also similar to that reported in Sweden as a whole.38 For example, for 1998 to 2003, the incidence rate per 100 000 women (aged 70–79 yr) was 73 cases in Sweden and 68 cases in our analytical cohort.
Limitations
The information on hormone replacement therapy was self-reported, which may have led to some degree of nondifferential misclassification. However, self-reporting of hormone replacement therapy has been shown to be highly comparable with prescription data,17 and the prevalence of hormone replacement therapy in our study was similar to that previously reported from Sweden for that period.36 Given the length of follow-up in our study, and given that the exposure was assessed only once, it is likely that the participants changed their use of hormone replacement therapy. We used several sensitivity analyses to evaluate whether this may have biased our findings, and the results did not depart substantially from those obtained in the main analysis. A second limitation was the lack of information on preparation, dose and route of administration of the hormone replacement therapy. Third, the cases were identified by register diagnosis, which may not have been entirely correct. However, the Swedish National Patient Register has been shown to be highly accurate for the diagnosis of acute pancreatitis,19 and this accuracy should not differ by use of hormone replacement therapy. To minimize misclassification with other exocrine pancreatic diseases, we used such diseases (including cancer) as censoring events. Finally, although we adjusted for several potential confounders, there may have been unmeasured or residual confounding.
Conclusion
Use of postmenopausal hormone replacement therapy was associated with increased risk of acute pancreatitis. The role of the preparation, dose and route of administration of hormone replacement therapy merits further investigation. If these findings are confirmed by other studies, the risk of acute pancreatitis should be considered when hormone replacement therapy is prescribed.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Viktor Oskarsson was involved in study conception and design, statistical analysis, interpretation of data, drafting of the manuscript and approval of the final manuscript. Nicola Orsini was involved in study conception and design, statistical analysis, interpretation of data, critical revision of the manuscript for important intellectual content and approval of the final manuscript. Omid Sadr-Azodi was involved in study conception and design, interpretation of data, critical revision of the manuscript for important intellectual content and approval of the final manuscript. Alicja Wolk was involved in study conception and design, acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content and approval of the final manuscript.
Funding: This study was funded by research grants from the Swedish Research Council/Committee for Infrastructure (Dnr. 2011-6262) and the Board of Research at Karolinska Institutet (Distinguished Professor Award; Dnr. 2368/10-221) to Alicja Wolk; from the Board of Postgraduate Education at Karolinska Institutet (Clinical Scientist Training Program; Dnr. 3023/11-225) to Viktor Oskarsson; from the Strategic Research Program in Epidemiology at Karolinska Institutet (Young Scholar Award; Dnr. 7340/2012) and the Swedish Society of Medicine (SLS-250271) to Nicola Orsini; and from the Swedish Society of Medicine (SLS-248111) and the Centre for Clinical Research Sörmland (DLL-302851) to Omid Sadr-Azodi. All work was done independent of the financial support.
References
- 1.Steer M. Pancreatitis severity: Who calls the shots? Gastroenterology 2002;122:1168–72 [DOI] [PubMed] [Google Scholar]
- 2.Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med 2006;354:2142–50 [DOI] [PubMed] [Google Scholar]
- 3.Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol 2005;39:709–16 [DOI] [PubMed] [Google Scholar]
- 4.Nitsche C, Maertin S, Scheiber J, et al. Drug-induced pancreatitis. Curr Gastroenterol Rep 2012;14:131–8 [DOI] [PubMed] [Google Scholar]
- 5.Blake WE, Pitcher ME. Estrogen-related pancreatitis in the setting of normal plasma lipids: case report. Menopause 2003;10:99–101 [DOI] [PubMed] [Google Scholar]
- 6.Trenque-Tessereau MG, Picot C, Herment N, et al. Combined estradiol/gestodene and acute pancreatitis. Ann Pharmacother 2005;39:1953–4 [DOI] [PubMed] [Google Scholar]
- 7.Glueck CJ, Lang J, Hamer T, et al. Severe hypertriglyceridemia and pancreatitis when estrogen replacement therapy is given to hypertriglyceridemic women. J Lab Clin Med 1994;123:59–64 [PubMed] [Google Scholar]
- 8.Glueck CJ, Scheel D, Fishback J, et al. Estrogen-induced pancreatitis in patients with previously covert familial type V hyperlipoproteinemia. Metabolism 1972;21:657–66 [DOI] [PubMed] [Google Scholar]
- 9.Isley WL, Oki J. Estrogen-induced pancreatitis after discontinuation of concomitant medroxyprogesterone therapy. Am J Med 1997; 102:416–7 [DOI] [PubMed] [Google Scholar]
- 10.Ruman J, Brenner S, Sauer MV. Severe hypertriglyceridemia and pancreatitis following hormone replacement prior to cryothaw transfer. J Assist Reprod Genet 2002;19:94–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidoff F, Tishler S, Rosoff C. Marked hyperlipidemia and pancreatitis associated with oral contraceptive therapy. N Engl J Med 1973;289:552–5 [DOI] [PubMed] [Google Scholar]
- 12.Bank S, Marks IN. Case reports. Hyperlipaemic pancreatitis and the pill. Postgrad Med J 1970;46:576–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mungall IP, Hague RV. Pancreatitis and the pill. Postgrad Med J 1975;51:855–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker WA. Estrogen-induced pancreatitis. Clin Pharm 1983; 2:75–9 [PubMed] [Google Scholar]
- 15.Tetsche MS, Jacobsen J, Norgaard M, et al. Postmenopausal hormone replacement therapy and risk of acute pancreatitis: a population-based case–control study. Am J Gastroenterol 2007; 102:275–8 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki R, Rylander-Rudqvist T, Ye W, et al. Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer 2006;119:1683–9 [DOI] [PubMed] [Google Scholar]
- 17.Banks E, Beral V, Cameron R, et al. Agreement between general practice prescription data and self-reported use of hormone replacement therapy and treatment for various illnesses. J Epidemiol Biostat 2001;6:357–63 [DOI] [PubMed] [Google Scholar]
- 18.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razavi D, Ljung R, Lu Y, et al. Reliability of acute pancreatitis diagnosis coding in a national patient register: a validation study in Sweden. Pancreatology 2011;11:525–32 [DOI] [PubMed] [Google Scholar]
- 20.Cologne J, Hsu WL, Abbott RD, et al. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology 2012;23:565–73 [DOI] [PubMed] [Google Scholar]
- 21.Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J 2011; 11:1–29 [Google Scholar]
- 22.Kleinbaum DG, Klein M. Survival analysis: a self-learning text. 2nd ed New York (NY): Springer; 2005 [Google Scholar]
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99 [DOI] [PubMed] [Google Scholar]
- 24.Oskarsson V, Sadr-Azodi O, Orsini N, et al. Vegetables, fruit and risk of non–gallstone-related acute pancreatitis: a population-based prospective cohort study. Gut 2013;62:1187–92 [DOI] [PubMed] [Google Scholar]
- 25.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349: 523–34 [DOI] [PubMed] [Google Scholar]
- 26.Sadr-Azodi O, Orsini N, Andren-Sandberg A, et al. Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study. Am J Gastroenterol 2013;108:133–9 [DOI] [PubMed] [Google Scholar]
- 27.Sadr Azodi O, Orsini N, Andren-Sandberg A, et al. Effect of type of alcoholic beverage in causing acute pancreatitis. Br J Surg 2011;98:1609–16 [DOI] [PubMed] [Google Scholar]
- 28.Sadr-Azodi O, Andren-Sandberg A, Orsini N, et al. Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut 2012;61:262–7 [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Beral V, Balkwill A, et al. Gallbladder disease and use of transdermal versus oral hormone replacement therapy in postmenopausal women: prospective cohort study. BMJ 2008; 337:a386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossouw JE, Cushman M, Greenland P, et al. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the Women’s Health Initiative trials of hormone therapy. Arch Intern Med 2008;168:2245–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindkvist B, Appelros S, Regner S, et al. A prospective cohort study on risk of acute pancreatitis related to serum triglycerides, cholesterol and fasting glucose. Pancreatology 2012;12:317–24 [DOI] [PubMed] [Google Scholar]
- 32.Andrén-Sandberg A, Hoem D, Backman PL. Other risk factors for pancreatic cancer: hormonal aspects. Ann Oncol 1999;10 (Suppl 4):131–5 [PubMed] [Google Scholar]
- 33.Pousette A, Fernstad R, Haggmark A, et al. The estrogen binding protein in human pancreas: concentrations in subcellular fractions of normal pancreatic tissue, in duodenal juice during pancreatic stimulation and in peripheral serum in normal and pathological conditions. J Steroid Biochem 1988;29:423–7 [DOI] [PubMed] [Google Scholar]
- 34.Blevins GT, Jr, McCullough SS, Wilbert TN, et al. Estradiol alters cholecystokinin stimulus-response coupling in rat pancreatic acini. Am J Physiol 1998;275:G993–8 [DOI] [PubMed] [Google Scholar]
- 35.Saluja AK, Lerch MM, Phillips PA, et al. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol 2007;69: 249–69 [DOI] [PubMed] [Google Scholar]
- 36.Li C, Samsioe G, Lidfelt J, et al. Important factors for use of hormone replacement therapy: a population-based study of Swedish women. The Women’s Health in Lund Area (WHILA) Study Menopause 2000;7:273–81 [PubMed] [Google Scholar]
- 37.Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst 1999;91:1131–7 [DOI] [PubMed] [Google Scholar]
- 38.Sandzén B, Rosenmuller M, Haapamaki MM, et al. First attack of acute pancreatitis in Sweden 1988–2003: incidence, aetiological classification, procedures and mortality — a register study. BMC Gastroenterol 2009;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]