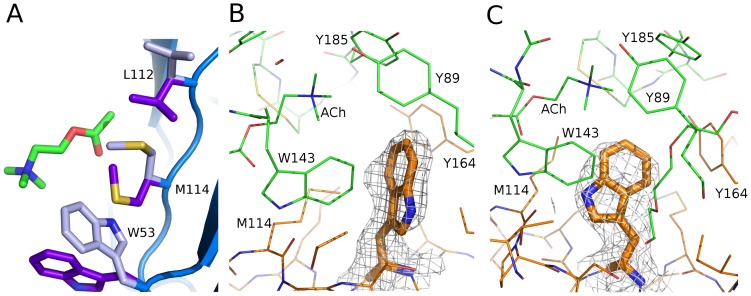
Figure 3. Two conformations of Trp53, Leu112 and Met114.
(A) On the complementary side of the interface, three residues near ACh adopt two distinct sets of conformations (shown in purple and light-blue sticks, respectively), at some interfaces occurring separately and at other interfaces with both conformations occurring as shown here. (B) and (C) Trp53 is shown in stick representation in the two different orientations observed, in interfaces where distinct orientations are seen. Principle side carbon atoms are colored green, while complementary side carbon atoms are orange. A mesh is shown in each case, corresponding to a partial omit map shown at 1ó and carved at 2 Å around Trp53. The partial omit map was generated using PHENIX by refining the structure after changing all Trp53 residues to alanine, thus alleviating side-chain orientation bias for this residue. (B) In one possible orientation, the Trp53 side-chain nitrogen atom is pointing “away” from Met114 with Trp53 and Trp143 aligned for T-type ππstacking. (C) In the other conformation, which is favored when a PEG400 molecule is present nearby, the Trp53 side-chain nitrogen atom is pointing towards Met114 and can form a hydrogen bond to the backbone carbonyl oxygen atom of this residue.