Figure 1. Evolving Cancer Stem Cell niche interactions during tumor progression.
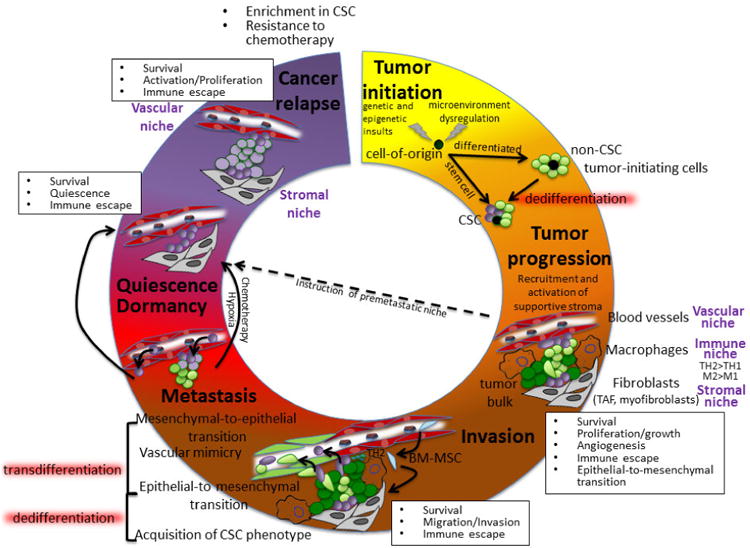
The tumor cell-of origin may initially display CSC features or CSC may appear during tumor progression. Complex interactions between all components of the microenvironment and CSCs organize distinct niches that govern tumor proliferation, immune escape, invasion, metastasis, dormancy and cancer relapse. CSCs have been located in close relationship with two distinct niches: the stroma and the vasculature. Both niches have been shown to play a critical role in regulating CSC phenotypes and initiate invasive, metastatic or dormant behaviors. The immune infiltrate plays also critical roles, modulating these niches or directly interacting with CSC. Circulating BM-MSCs can be recruited at the primary site of tumor, where they can contribute both directly and indirectly to the primary tumor niche or can participate in the establishment of the metastatic niche. CSC transdifferentiation has been suggested based on CSC acquisition of endothelial (vascular mimicry) or mesenchymal (epithelial-to-mesenchymal/mesenchymal-to-epithelial transitions) traits, to support tumor growth or invasiveness. The involvement of the microenvironment in the possible dedifferentiation of non-CSCs to a CSC phenotype has also been suggested. Acquisition of CSC features is often partially reminiscent of embryonic phenotypes and possible dedifferentiation process may involve signaling routes exploited by induced pluripotency. The metastatic process is highly inefficient, but instruction of a premetastatic niche by the primary tumor and acquisition of a CSC phenotype by invasive cells may favor survival and engraftment of circulating cancer cells in secondary niches. While the osteoblast niche seems to regulate the fate of leukemic CSCs for cancer relapse, the vascular niche has been involved in establishment and exit of breast cancer dormancy.
