Abstract
Background
Chronic obstructive pulmonary disease (COPD), is a very common disease of respiratory system. An increasing number of clinical trials on Yupingfeng formula in the management of stable COPD have been performed. However, the evidence base for it remains unknown. This review aims at assessing the efficacy, and safety of modified Yupingfeng formula in the treatment of stable COPD through a systematic review of all available randomized controlled trials.
Materials and Methods
Literature retrieval was conducted using four English databases (CENTRAL, PubMed, EMBASE, and ISI Web of Science), and four Chinese databases (CBM, CNKI, VIP, and WANFANG), from respective inception to January 2013, and supplemented with a manual search. Review authors independently extracted the trial data, and assessed the quality of each trial. Methodological quality was assessed by Cochrane risk of bias and Jadad's scale. The following outcomes were evaluated: (1) lung function; (2) 6-minute walk distance (6MWD); (3) effective rate; (4) serum levels of IgA, IgG and IgE; and (5) adverse events. Data were analyzed using STATA 12.0 software.
Results
A total of nine studies involving 660, stable COPD patients were identified. Patients from all studies included in this review were randomized to receive Yupingfeng formula combined with Western medications in comparison with Western medications. In general, the methodological quality of the included trials was poor. The results of this systematic review indicates that, compared with Western medications alone, the use of Yupingfeng formula, if combined with Western medications could significantly improve FEV1 (WMD = 0.30L; 95%CI: 0.19, 0.42), FEV1/FVC ratio (SMD = 0.69; 95%CI: 0.48, 0.91), 6MWD (WMD = 31.73m; 95% CI: 19.29, 44.17), and effective rate (RR = 1.24; 95% CI: 1.10, 1.41), and increase the serum levels of IgA (WMD = 0.25; 95%CI: 0.16, 0.34) and IgG (WMD = 1.10; 95%CI: 0.53, 1.68), but no difference was found in the serum IgE levels (WMD = 0.47; 95%CI: −0.32, 1.27) between the two groups. No serious adverse events were reported.
Conclusions
Within the limitations of this systematic review, we may conclude that compared with Western medications alone, Yupingfeng formula, when combined with Western medications can provide more benefits for patients with stable COPD, without any serious adverse reactions being identified. However, these benefits need to be further confirmed through high-quality prospective placebo-controlled trials that should be strictly conducted in accordance with methodological principles and procedures.
Keywords: Yupingfeng formula, chronic obstructive pulmonary disease, Systematic review
Introduction
Chronic obstructive pulmonary disease (COPD), is a common preventable disease characterized by persistent airflow limitation that is usually progressive. Although it can be properly managed, [COPD], is said to be one of the leading cause with high morbidity and mortality rates globally, resulting into an economic, and social burden that is both substantial and increasing. It ranked sixth as a major cause of death in 1990, and it is estimated that COPD will become the fourth leading cause of death globally by 2030. The prevalence and associated socio-economic burdens of COPD are projected to increase in the coming decades (Gold members, 2013).
Conventional therapy for stable COPD is aimed at relieving symptoms, reducing long-term lung function decline, preventing exacerbations, and improving health status and exercise tolerance. The management steps of COPD includes smoking cessation, oxygen therapy, physical activity, pulmonary rehabilitation, nutritional supplementation, vaccinations, and the use of inhaled corticosteroids, long-acting bronchodilators, or combination of corticosteroid and bronchodilator therapy if necessary (Qaseem et al., 2011). However, COPD is a progressive disease leading to a decrease in lung function over time with the best available care. Till date, none of the existing Western medications for COPD has been conclusively shown to modify the long-term decline in lung function (Gold members, 2013). Unsatisfactory treatment response from conventional Western drugs contributes to the increasing popularity of Traditional Chinese Medicine (TCM).
TCM has its potential advantages different from modern medicines, and is recognized as an attractive alternative to conventional Western medicine. There is a long history in the use of TCM in treating respiratory diseases, particularly in China, Japan, and other Asian countries (Sorkness et al., 2009). According to the TCM theory, COPD should be classified in the category of syndrome characterized by dyspnea and cough. From the standpoint of TCM patients, COPD usually manifest with Qi-deficiency syndrome (Fu et al., 2007). In TCM, Qi is one of the most important substances in the body, and it spreads over the whole body via its dispersing and descending functions. When the Qi is too weak to control the pores and protect the body, the patient is more susceptible to catching a cold (Yang et al., 2010; Dowie et al., 2009), which commonly leads to acute exacerbations of COPD. Therefore, the treatment principle of COPD should focus on nourishing Qi. Previous clinical studies have demonstrated ginseng or combined with other herbs can improve the lung function of patients with COPD (An et al., 2011).
Yupingfeng formula (Gyokuheifu-san in Japanese, Jade Windscreen Powder in English), originally recorded in the book Danxi Xinfa, is a classical TCM formula consisting of three herbal medicines, i.e. Huang Qi (Astragalus membranaceus); Bai Zhu (Rhizoma atractylodis macrocephalae); and Fang Feng (Radix saposhnikoviae). Huang Qi is a powerful medicine for strengthening Qi of spleen and lung to activate body energy as well as to protect the body form external pathogens. Bai Zhu strengthens spleen, which is the source of Qi. Fang Feng can benefit the defensive Qi, and consolidate the exterior. By combining these three herbal medicines, Yupingfeng formula has the potential to invigorate Qi and consolidate the superficial resistance, which may increase a patient's ability to protect himself from invasion by external pathogenic influence (Makino et al., 2004). It is frequently used for treating spontaneous sweating, aversion to wind, weakness and a susceptibility to catching colds. Moreover, it has been widely prescribed for hundreds of years to prevent or treat respiratory tract diseases, such as perennial allergic rhinitis, respiratory infections, bronchial asthma, and COPD when present as Qi-deficiency syndrome (Fang et al., 2005). Increasing efforts have been directed towards seeking for relevant scientific evidence, and an increasing number of clinical trials on Yupingfeng formula in the management of COPD have been performed, but the findings have not yet been systematically summarized.
The purpose of the current systematic review was to assess the efficacy and safety of modified Yupingfeng formula in the treatment of COPD through a systematic collection of evidence from randomized controlled trials (RCTs). It was expected that, this systematic review could provide more evidence-based information for real-world clinical practice.
Methods
Criteria for considering studies for this review
Types of studies
Only [RCTs], were eligible for this review, irrespective of blinding. Quasi-randomized and nonrandomized controlled trials were not considered.
Types of participants
We reviewed studies of adults with COPD defined as progressive chronic air-flow limitation evident by spirometry with a post-bronchodilator FEV1/FVC ratio < 70%, regardless of smoking history. Patients were in a clinically stable state at the start of the study, without recent exacerbation, hospitalization or, any need for antibiotics nor systemic corticosteroids. Patients did not have any clinical features of asthma.
Types of interventions
A comparative study between Yupingfeng formulas or, when combined with Western medications, and placebo; no treatment or, same Western medications as controls were included. In clinical practice of TCM, it is common to modify original formula by adding or substituting herbs in accordance with a patient's syndrome to enhance the efficacy of the original formula. Thus, any form of administration of Yupingfeng formula or its modified prescription such as decoction, capsule, tablet, pill, and powder was acceptable for inclusion in the review. Papers were excluded when Chinese herbs or other TCM therapies were used in the control group.
Types of outcome measures
The primary outcome measure was lung function.
Secondary outcomes included: (1) exercise capacity (6-minute walk distance, 6MWD); (2) effective rate defined as the symptoms scores reduced rate (cough, cough-up phlegm, dyspnea, wheezing or other lung symptoms) ≥ 30% according to the Chinese criteria “Guiding Principle of Clinical Research on New Drugs of TCM” (Zhen et al., 2002; Chen et al., 2010; Bian et al., 2008); (3) serum levels of IgA, IgG and IgE; and (4) adverse events.
Search methods for identification of studies
The retrieval of trials was performed in the Cochrane Central Register of Controlled Trials (CENTRAL), Pub Med, EMBASE, ISI Web of Science, Chinese Biomedical Database (CBM); Chinese National Knowledge Infrastructure (CNKI), Chinese Scientific and Technological Periodical Database (VIP), and WANFANG Database. All databases were searched from their respective inception dates, to January 2013. Searches were conducted to identify all relevant studies regardless of language or publication status. The search strategy was constructed by using a combination of subject headings and text words relating to the use of Yupingfeng formula in the treatment of patients with stable COPD. The search terms used in PubMed were [(((((Yupingfeng[Title/Abstract]) OR Yu Ping Feng [Title/Abstract]) OR Gyokuheifu [Title/Abstract]) OR Jade Windscreen [Title/Abstract]) OR “yupingfeng” [Supplementary Concept]) AND (“Pulmonary Disease, Chronic Obstructive"[Mesh] OR “COPD, Severe Early-Onset” [Supplementary Concept]) OR chronic obstructive pulmonary disease [Title/Abstract]) OR chronic obstructive lung disease [Title/Abstract]) OR chronic obstructive airway disease [Title/Abstract]) OR chronic airflow obstruction [Title/Abstract]) OR obstructive airway disease [Title/Abstract]) OR COPD [Title/Abstract]) OR COAD [Title/Abstract]) OR chronic bronchitis [Title/Abstract]) OR emphysema [Title/Abstract]) AND ((“Randomized Controlled Trial” [Publication Type] OR “Randomized Controlled Trials as Topic” [Mesh]) OR random* [Title/Abstract])]. Various combinations of the terms were used, depending on the database searched. We also searched on-line web sites including journal websites and databases of ongoing trials.
In addition, a manual search of ten leading TCM journals was performed to identify other potentially relevant studies. Meanwhile, we also searched the reference lists of review articles and identified RCTs for any possible titles matching the inclusion criteria. Furthermore, we contacted experts in this field, and relevant pharmaceutical companies for other unpublished studies.
Selection of studies
Two review authors (Yunqing Zhong and Xiufeng Wang), independently selected trials for inclusion by scanning the titles, abstract sections, and keywords of each study retrieved and the full-text articles if necessary. Agreement between review authors for inclusion of studies was recorded.
Data extraction and management
To avoid bias in the data extraction process, the review authors (Bing Mao and Wei Zhou) independently extracted data using a piloted data extraction form and compared the results. They were not blinded to study authors, institutions, or journals of publication. Details of participants, severity of COPD, COPD duration, study design, interventions, outcome measures and adverse events of each study were extracted. Any lack of information was supplemented by correspondence with the original principal investigators. Each entry was double-checked by both reviewers. All review authors participated in resolving discrepancies until a consensus was reached.
Quality assessment
Risk of bias was independently assessed by two review authors (Jie Min and Hongli Jiang), according to the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins et al., 2013) across six domains, i.e. sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. Each domain was classified as having either a high, low, or unclear risk of bias. We also used the Jadad scoring system for quality assessment, which evaluates a study in terms of the description of randomization, blinding, and dropouts. The Jadad scale is a 5-point scale for assessing the quality of randomized trials in which three points or more indicate superior quality (Jadad et al., 1996). Discrepancies were resolved by consensus. If required, a third party (Bing Mao) would be consulted. To avoid selection bias, no study was rejected because of these quality criteria.
Data synthesis
All calculations were conducted using STATA software (STATA version 12.0, StataCorps, College Station, Texas, USA). We performed the meta-analysis by pooling homogeneous studies. For dichotomous data, we presented results as relative risk (RR), with 95% confidence interval (CI). For continuous data, we calculated weighted mean difference (WMD) if outcomes were measured in a same way among trials or standardized mean difference (SMD) if outcomes were measured by different methods among trials. Heterogeneity between the trials was analyzed by Cochrane's Q test, and potential reasons for heterogeneity were also explored. If the analysis showed low heterogeneity (P ≥ 0.10 and I2 ≤ 50%), data were synthesized using a fixed-effect model. Otherwise, a random-effects model was applied. Publication bias was assessed by Egger's and Begg's statistical tests and funnel plot if the meta-analysis included more than 10 trials (Sedgwick P., 2013). A two-tailed P value less than 0.05 was considered statistically significant.
Sensitivity analysis
We used STATA's METANINF command to investigate whether the exclusion of any one study would significantly change the pooled estimate, that is, whether the pooled point estimate with one study excluded would lie outside the 95% CI of the pooled estimate with all studies included. If the sensitivity analysis was close to the results of the complete study analysis, this strengthened the level of confidence.
Results
Description of studies
Figure 1: show the paper selection process, and exclusion reasons. A total of 76, potentially eligible citations were identified in the initial search, of which 45, were excluded because those were not relevant to the topic. Based on title and abstract, other 13, articles were further excluded for the following reasons: 2, review articles, and 11, animal studies. Two studies that were pre-post comparisons with no control group were also excluded. Of the remaining 16, articles, two additional trials which enrolled patients with acute exacerbations of COPD were further excluded. After careful examination of the retrieved articles, 4 were also excluded because other Chinese herbs were used as controls. One trial (Li et al., 2010), was presented in two papers, therefore this was considered as one study. Finally, a total of 9 RCTs (Huang et al., 2005; Zhao et al., 2009; Li et al., 2010; Xu J., 2010; Xu X.H., 2010; Zhang et al., 2011; Liao et al., 2011; Liu et al., 2012; Wang et al., 2012) that satisfied the inclusion criteria were included. All of the included studies compared Yupingfeng formula combined with conventional Western medications (therapy A) with the same conventional Western medications alone (therapy B).
Figure. 1.
Flow diagram showing the stages of the identification of studies for the meta-analysis.
Characteristics of included trials
The main characteristics of the included trials are listed in Tables 1 and 2. All of the identified studies involving a total of 660, stable COPD patients were conducted in China, and all articles were written in Chinese language. None of these was designed as a multicenter study. All trials were published in scientific journals, no unpublished dissertations or conference papers were included. All nine trials were parallel-design, randomized controlled trials. The publication years of these studies ranged from 2005, to 2012. The number of subjects included in each study ranged from 40–100. Eight studies (Huang et al., 2005; Zhao et al., 2009; Li et al., 2010; Xu J., 2010; Zhang et al., 2011; Liao et al., 2011; Liu et al., 2012; Wang et al., 2012), enrolled both men and women, and the other study (Xu, X.H., 2010) conducted exclusively in men. Four studies (Zhao et al., 2009; Zhang et al., 2011; Liao et al., 2011; Liu et al., 2012) did not specify the severity classification of airflow limitation in COPD subjects. Two trials (Xu, X.H., 2010; Zhang et al., 2011) reported withdrawals, and dropouts. One (Xu, X.H., 2010) did not provide reasons, while the other (Zhang et al., 2011), explained by the following reasons: 2 patients in therapy A group suffered from COPD exacerbations, and 5 could not tolerate TCM; 4 patients in therapy B group withdrew due to COPD exacerbations, and 1 experienced a cerebrovascular accident.
Table 1.
Patient characteristics of the included studies
| First author, reference, (year) |
Location of Hospital |
Source of patients |
No. of Participants |
No. of Male/Female |
Age (Mean ± SD or age range, years) |
Severity of COPD | COPD Duration (Mean ± SD or range, years) |
| Huang et al., 2005 | Guangdong, China | Outpatients | T: 32 C: 31 |
T: 21/11 C: 23/8 |
T: 69.5±11.8 C: 68.8±10.6 |
T: II 17, III 15 C: II 18, III 13 |
T: 8.5±3.2 C: 8.3±3.5 |
| Zhao et al., 2009 | Guangdong, China | Inpatients | T: 45 C: 30 |
T&C: 50/25 | T&C: 67.3±5.2 | NS | T&C: 3–12 |
| Li et al., 2010 | Beijing, China | Outpatients | T: 29 C: 20 |
T: 19/10 C: 14/6 |
T: 62±8.61 C: 61.12±8.12 |
T: Moderate 20, Severe 9 C: Moderate 16, Severe 4 |
NS |
| Xu J, 2010 | Zhejiang, China | NS | T:50 C:50 |
T&C: 71/29 | T&C: 45–79 | Mild 54, Moderate 36, Severe 10 | T&C: 3–41 |
| Xu XH, 2010 | Jiangsu, China | NS | T:45*/44# C: 45 |
T: 44/0 C: 45/0 |
T&C: 60.1±7.7 | T&C: II–III | T&C: 10.1±4.2 |
| Zhang et al., 2011 | Fujian, China | NS | T: 50*/43# C: 50*/45# |
T: 36/7 C: 39/6 |
T: 56.20±7.12 C: 55.21±7.01 |
NS | T: 16.02±8.96 C: 15.26±9.10 |
| Liao et al., 2011 | Hubei, China | NS | T: 30 C: 33 |
T: 21/9 C: 23/10 |
T: 37–72 C: 39–70 |
NS | T: 5–33 C: 5–30 |
| Liu et al., 2012 | Henan, China | NS | T: 20 C: 20 |
T: 19/1 C: 18/2 |
T: 66±11 C: 64±9 |
NS | T: 20±10 C: 18±9 |
| Wang et al., 2012 | Sichuan, China | NS | T: 40 C: 40 |
NS | T&C: 55–80 | T&C: II–IV | NS |
T: treatment; C: control; NS=not specified; *: number of subjects randomized; #: number of subjects analyzed; COPD: chronic obstructive pulmonary disease; SD: standard deviation.
Table 2.
Other detailed characteristics of the included studies
| First author, reference, (year) |
Study design | Methods of random process |
Interventions (Trial group) |
Interventions (Control group) |
Duration/Follow-up | Outcome measures |
Adverse events | Similarity at baseline |
ITT | Jadad score |
| Huang et al., 2005 | Randomized single-blind controlled trial |
Unclear |
|
CWM | 3 months/6 months |
FEV1, FEV1/FVC, 6MWD |
T: dry mouth and mild abdominal distension (2 cases) C: loss of appetite (1 case) |
Similar | No | 1 |
| Zhao et al., 2009 | Randomized controlled trial |
Unclear |
|
CWM (continuous low-flow oxygen therapy, prescriptions for eliminating phlegm, etc) |
12weeks/1 year |
Effective rate, FEV1 |
NS | Similar | No | 1 |
| Li et al., 2010 | Randomized controlled trial |
Random number table |
|
CWM (sustained-release aminophylline tablets, ambroxol hydrochloride tablets, and inhaled ipratropium bromide aerosol) |
90 days/NS | Effective rate, FEV1 |
NS | Similar | No | 2 |
| Xu J, 2010 | Randomized controlled trial |
Unclear |
|
CWM (prescriptions for facilitating expectoration, suppressing cough and relieving dyspnea) |
16 weeks/NS | FEV1/FVC, effective rate |
None | Similar | No | 1 |
| Xu XH, 2010 | Randomized controlled trial |
Random number table |
|
CWM (smoking cessation, respiratory muscle training, etc) |
3 months/NS | Effective rate, IgA, IgG, IgM |
None | Similar | No | 2 |
| Zhang et al., 2011 | Randomized controlled trial |
Unclear |
|
Seretide 50/500 | 24weeks/NS | 6MWD | NS | Similar | No | 2 |
| Liao et al., 2011 | Randomized single-blind controlled trial |
Random number table |
|
CWM (health education, respiratory muscle training, etc) |
3 months/NS | FEV1, FEV1/FVC, effective rate, 6MWD, IgA, IgG, IgM |
None | Similar | No | 2 |
| Liu et al., 2012 | Randomized controlled trial |
Unclear |
|
CWM (smoking cessation, respiratory muscle training, sustained-release aminophylline tablets, ambroxol hydrochloride tablets, and inhaled ipratropium bromide aerosol) |
90 days/NS | FEV1, FEV1/FVC, effective rate |
NS | Similar | No | 1 |
| Wang et al., 2012 | Randomized controlled trial |
Unclear |
|
CWM (health education, respiratory muscle training, facilitating expectoration, suppressing cough and relieving dyspnea) |
6 months/1 year |
FEV1, FEV1/FVC, effective rate, 6MWD, IgA, IgG, IgM |
None | Similar | No | 1 |
NS=not specified; CWM, conventional Western medicine; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; 6MWD, 6-min walk distance
Methodological quality
Assessment of risk of bias
All the included studies claimed “randomization”, but only three studies (Li et al., 2010; Xu X.H., 2010; Liao et al., 2011), clearly described the method of randomization (a random number table). Thus, the information of sequence generation was adequate for three studies (Li et al., 2010; Xu X.H., 2010; Liao et al., 2011) at low risk of bias (yes) and inadequate for 6 studies (Huang et al., 2005; Zhao et al., 2009; Xu J, 2010; Zhang et al., 2011; Liu et al., 2012; Wang et al., 2012), with uncertain risk of bias (unclear). No trials described allocation concealment, and the domain was judged as ‘unclear’ in all studies. Blinding procedures (single-blind) were mentioned in two studies (Huang et al., 2005; Liao et al., 2011), but no trials provided information about the blinding of either participants or investigators after assignment to interventions. Two studies (Xu X.H., 2010; Zhang et al., 2011), provided data on withdrawals, but only one (Zhang et al., 2011), described the reasons. No trials described intention-to-treat analyses (ITT), or the method of assessing compliance. No studies mentioned a previous published protocol, but two studies (Liao et al., 2011; Wang et al., 2012) included all expected outcomes. Thus, selective outcome reporting was at low risk of bias in 2 studies (Liao et al., 2011; Wang et al., 2012) and at uncertain risk of bias in 7 studies (Huang et al., 2005; Zhao et al., 2009; Li et al., 2010; Xu J., 2010; Xu X.H., 2010; Zhang et al., 2011; Liu et al., 2012). Assessment of other sources of bias included early stopping for benefit and baseline imbalance. The baseline characteristics of the trial and control groups in the included trials were comparable. But none of the studies described any pre-calculated sample size, and information on early stopping was insufficient. Overall, the domain was rated as “unclear” in all studies. Detailed information is shown in Table 3.
Table 3.
Assessment of risk of bias in the included studies
| First author, reference, (year) |
Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | |
| Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data addressed |
Free of Selective outcome reporting |
Free of Other sources of bias |
|
| Huang et al., 2005 | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Unclear |
| Zhao et al., 2009 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Li et al., 2010 | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Xu J, 2010 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Xu XH, 2010 | Yes | Unclear | Unclear | Unclear | No | Unclear | Unclear |
| Zhang et al., 2011 | Unclear | Unclear | Unclear | Unclear | No | Unclear | Unclear |
| Liao et al., 2011 | Yes | Unclear | Yes | Yes | Unclear | Yes | Unclear |
| Liu et al., 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Wang et al., 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear |
Yes, low risk of bias; No, high risk of bias; Unclear, uncertain risk of bias.
Assessment of Jadad's scale
Through reading the full text, all nine studies' level of evidence was graded scores lower than three according to the Jadad quality score. Four studies (Li et al., 2010; Xu X.H., 2010; Zhang et al., 2011; Liao et al., 2011) were rated with a score of two, and five studies (Huang et al., 2005; Zhao et al., 2009; Xu J., 2010; Liu et al., 2012; Wang et al., 2012) with a score of 1. In general, the methodological quality of the included trials was poor (Table 2).
Effects of interventions
FEV1
A total of six trials (Huang et al., 2005; Zhao et al., 2009; Li et al., 2010; Liao et al., 2011; Liu et al., 2012; Wang et al., 2012) involving 370, stable COPD patients provided data on the FEV1. The random-effects model was used because of intertribal heterogeneity (variability in the intervention effects across studies) of the results of trials (I2 = 51.4%, P = 0.067). A pooled analysis of the 6 trials showed that therapy A improved the FEV1 to a greater extent than therapy B (WMD = 0.30L; 95%CI: 0.19, 0.42) (Figure 2).
Figure 2.
Meta-analysis of FEV1 in randomized controlled trials comparing Yupingfeng formula plus Western medications with the same Western medications alone in the treatment of stable COPD. FEV1: forced expiratory volume in 1 second.
FEV1/FVC ratio
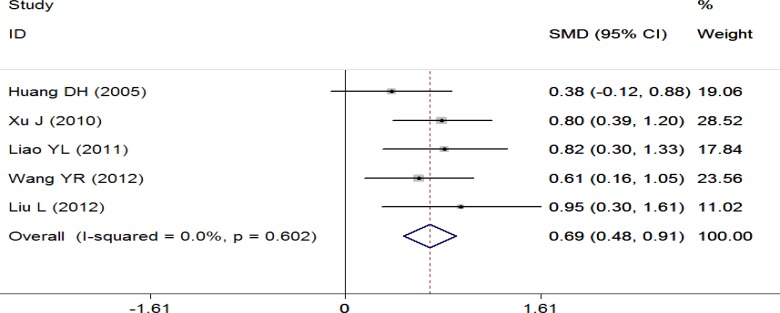
This outcome was reported in five trials (Huang et al., 2005; Xu J., 2010; Liao et al., 2011; Liu et al., 2012; Wang et al., 2012) which included 346 patients. A pooled analysis was conducted using the fixed-effect model (I2 = 0.0%, P = 0.602). Statistically significant differences were noted between the two groups, and these results suggested that therapy A significantly improved the FEV1/FVC ratio compared with therapy B (SMD = 0.69; 95%CI: 0.48, 0.91) (Figure 3).
Figure 3.
Meta-analysis of FEV1/FVC ratio in randomized controlled trials comparing Yupingfeng formula plus Western medications with the same Western medications alone in the treatment of stable COPD. FVC: forced vital capacity.
6MWD
A pool analysis of 4 trials (Huang et al., 2005; Zhang et al., 2011; Liao et al., 2011; Wang et al., 2012) was performed using a fixed-effect model (I2 = 0.0%, P = 0.401). Results indicated that 6MWD was significantly improved in therapy A group compared with therapy B group (WMD = 31.73m; 95% CI: 19.29, 44.17) (Figure 4).
Figure 4.
Meta-analysis of 6MWD in randomized controlled trials comparing Yupingfeng formula plus Western medications with the same Western medications alone in the treatment of stable COPD. 6MWD: 6 minute walk distance.
Effective rate (the symptoms scores reduced rate)
Seven trials (Zhao et al., 2009; Li et al., 2010; Xu J., 2010; Xu X.H., 2010; Liao et al., 2011; Liu et al., 2012; Wang et al., 2012) reported data on the outcome measure of effective rate, which was defined according to the criteria outlined in the “Guiding Principle of Clinical Research on New Drugs of Traditional Chinese Medicine” (Zhen et al., 2002). The random-effects model was used because of inter-tribal heterogeneity of the results of trials (I2 = 57.5%, P = 0.028). Results revealed significant differences between the two groups (P = 0.001), which was in favor of therapy A (RR = 1.24; 95% CI: 1.10, 1.41) (Figure 5).
Figure 5.
Meta-analysis of effective rate (the symptoms scores reduced rate) in randomized controlled trials comparing Yupingfeng formula plus Western medications with the same Western medications alone in the treatment of stable COPD.
Serum levels of IgA, IgG and IgE
Three (Xu X.H., 2010; Liao et al., 2011; Wang et al., 2012), out of the nine studies that compared therapy A, with therapy B reported serum IgA, IgG and IgE levels suitable for analysis. Further meta-analysis was conducted for the individual domains: IgA (WMD = 0.25; 95%CI: 0.16, 0.34); IgG (WMD = 1.10; 95%CI: 0.53, 1.68); and IgE (WMD = 0.47; 95%CI: −0.32, 1.27) (Fig. 6). The concentrations of serum IgA and IgG in stable COPD patients receiving therapy A were significantly higher than those receiving therapy B, and patients receiving therapy A were more likely to gain improvements in serum IgA, and IgG levels. But no difference was found in the serum IgE levels between the two groups, and there was no evidence that therapy A significantly altered the levels of serum IgE compared with therapy B.
Figure 6.
Meta-analysis of serum IgA, IgG and IgE levels in randomized controlled trials comparing Yupingfeng formula combined with Western medications with the same Western medications alone in the treatment of stable COPD.
Adverse events
One study (Huang et al., 2005) stated that two patients receiving therapy A experienced dry mouth and mild abdominal distension, and 1 subject receiving therapy B complained of loss of appetite. But all of these symptoms gradually disappeared after proper medical treatment. The remaining eight studies did not report any adverse events.
Publication bias
In general, if the number of studies is less than 10, a funnel plot is thought to be an unreliable method of investigating publication bias (Sedgwick, 2013). Due to the insufficient number of included studies, a formal assessment of reporting bias by visual inspection of a funnel plot was not possible.
Sensitivity analysis
As shown in Figure 2, the meta-analysis of FEV1 revealed a relatively high degree of heterogeneity across the studies. We used STATA's METANINF command to examine the influence of an individual study on the pooled estimate (WMD) by excluding each study in turn. For example, rerunning the analysis without the study (Li et al., 2010) changed the WMD from 0.30 (95%CI: 0.19, 0.42) to 0.37 (95%CI: 0.32, 0.42), thus the influence of this study on the estimated overall effect size was minor. The results were not materially changed, which suggested the stability of the results of the meta-analysis (Figure 7).
Figure 7.
Results of an influence analysis in which the meta-analysis of FEV1 is re-estimated omitting each study in turn. For example, rerunning the analysis without the study (Li et al., 2010) changed the weighted mean difference (WMD) from 0.30 (95%CI: 0.19, 0.42) to 0.37 (95%CI: 0.32, 0.42). The results were not materially changed, which suggested the stability of the results of the meta-analysis. The straight vertical lines at 0.30, 0.19 and 0.42 represent the WMD, upper and lower 95% CI of the complete study analysis, respectively.
Discussion
Yupingfeng formula, a well-known traditional Chinese medicine, commonly used to treat the diseases of the respiratory system. The aim of this study was to provide a comprehensive systematic review to assess the efficacy and safety of modified Yupingfeng formula for patients with stable COPD. The results of review indicate that compared with Western medications alone, the use of Yupingfeng formula combined with Western medications could significantly improve lung function, 6MWD, and effective rate (the symptoms scores reduced rate), and increase the serum IgA and IgG levels, but no difference was found in the serum IgE levels between the two groups. Our current results indicate that Yupingfeng formula could significantly enhance the humoral immune function through the increase of serum levels of IgA and IgG. Based on the results of this review, conventional Western drugs given in combination with orally administered Yupingfeng formula seem superior to conventional Western drugs alone for patients with stable COPD. Also, as no significant adverse events were observed in the included studies, Yupingfeng formula appears to be well tolerated, even when combined with conventional Western medicine.
These findings are consistent with previous data. Makino et al., (2005a) found that Yupingfeng formula could stimulate immune responses when the antigen had already invaded the body, and that it might consolidate the resistance of nasal mucosa to protect from ovalbumin (OVA) invasion. Another study by Makino et al., (2004) showed that Yupingfeng formula had preventive and curative effects on allergic rhinitis induced with Japanese cedar pollens in guinea pig. Song et al., (2013) observed that Yupingfeng powder exhibited anti-inflammatory and immunoregulatory effects in a rat model of chronic bronchitis, and time-dependent relationships in vitro. Moreover, pharmacological studies have showed that Yupingfeng formula as a holistic Kampo medicine might affect human homeostasis and constitution of human beings, and increase the immunological function of the body (Makino T., 2005b; Hong et al., 2011). The above pharmacological properties of Yupingfeng formula can partially explain the clinical benefits identified in this review.
It is noteworthy that this review has several limitations. Firstly, as the definition of COPD has been changing over the years (Chhabra S.K., 2009), differences in definition of the disease may bias our search results. Although a comprehensive search strategy was adopted, we still cannot guarantee that all eligible trials have been identified. And we were also unable to use formal methods to determine if there was any publication bias as too few studies were available. The possibility that there are unpublished studies or other published studies that were not indexed in the electronic databases we searched cannot be excluded, because negative or no significant findings are less likely to be published (Dirnagl et al., 2010). Secondly, the reporting of trial methods and procedures was unclear and insufficient. The majority of the 9 included studies suffered from methodological weaknesses based on the assessment of the Cochrane risk of bias and the Jadad's scale. The inadequacy of sequence generation, allocation concealment and blinding, and dropouts account were the major sources of potential bias. Low-quality trials are more likely to overestimate efficacy. Therefore, these results must be interpreted with caution. Thirdly, none of these studies was designed as a multicenter study. Most of the trials were of small sample size trials, and no studies estimated the sample size, which is essential for ensuring adequate sample size and statistical power to detecting clinical significant difference between interventions (Eng J., 2003; Scales et al., 2005). Fourthly, for the purpose of evaluating effects of therapies, the test intervention should be compared with placebo (Shah K.N., 2009). However, no placebo-controlled trial has studied the efficacy and safety of Yupingfeng formula for stable COPD. Finally, although the safety profile of Yupingfeng formula has been demonstrated in this systematic review, we still cannot be assured of its safety because the small sample sizes might have limited power to detect rare adverse events.
Although the results of this review may be limited by the low quality of the included trials, the findings are promising, and further investigation into the efficacy and safety of Yupingfeng formula is worthy of merit. The methodological quality of clinical trials on Yupingfeng formula in the treatment of stable COPD needs to be further improved in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement, and the publication of protocols should be encouraged (Schulz et al., 2010; Gagnier et al., 2006). In particular, large-scale, transnational cooperative, rigorously designed, multicenter, randomized, placebo-controlled, double-blind trials with explicit clinical and diagnostic criteria, sufficient duration of follow-up and description of all relevant clinical outcome measures are still warranted.
Conclusions
Within the limitations of this systematic review, we may conclude that compared with Western medications alone, combined Yupingfeng formula and Western medications can provide more benefits for patients with stable COPD, without any serious adverse reactions being identified. There is encouraging evidence suggesting that Yupingfeng formula may be a safe and effective herbal treatment option for patients with stable COPD. However, these benefits need to be further confirmed through high-quality prospective trials that should be strictly conducted in accordance with methodological principles and procedures.
Conflict of interests: No conflict of interests declared.
Acknowledgements
No grants or funding was provided for the performance of this study. Many thanks to Professor Guan-Jian Liu, a member of the Chinese Evidence-based Medicine/Cochrane Centre, West China Hospital, Sichuan University, for his valuable scientific suggestions in the search strategy. We also thanked the anonymous referees for their helpful comments and advice, which greatly improved the quality of this review.
References
- 1.An X, Zhang AL, Yang AW, Lin L, Wu D, Guo X, Shergis JL, Thien FC, Worsnop CJ, Xue CC. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respir Med. 2011;105:165–176. doi: 10.1016/j.rmed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Bian ZX, Moher D, Li YP, Wu TX, Dagenais S, Cheng CW, Li J, Li TQ. Appropriately selecting and concisely reporting the outcome measures of randomized controlled trials of traditional Chinese medicine. Zhong Xi Yi Jie He Xue Bao. 2008;6:771–775. doi: 10.3736/jcim20080801. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Chen M, Kang M, Xiong J, Chi Z, Zhang B, Fu Y. The design and protocol of heat-sensitive moxibustion for knee osteoarthritis: a multicenter randomized controlled trial on the rules of selecting moxibustion location. BMC Complement Altern Med. 2010;10:32. doi: 10.1186/1472-6882-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chhabra SK. Chronic obstructive pulmonary disease: Evolving definitions and their impact. The Indian Journal of Chest Diseases and Allied Sciences. 2009;51:135–137. [Google Scholar]
- 5.Dirnagl U, Lauritzen M. Fighting publication bias: introducing the Negative Results section. J Cereb Blood Flow Metab. 2010;30:1263–1264. doi: 10.1038/jcbfm.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowie S. Acupuncture. Edinburgh: Churchill Livingstone; 2009. Lung Qi deficiency; p. B62. [Google Scholar]
- 7.Eng J. Sample size estimation: how many individuals should be studied? Radiology. 2003;227:309–313. doi: 10.1148/radiol.2272012051. [DOI] [PubMed] [Google Scholar]
- 8.Fang SP, Tanaka T, Tago F, Okamoto T, Kojima S. Immunomodulatory effects of gyokuheifusan on INF-gamma/IL-4 (Th1/Th2) balance in ovalbumin (OVA)-induced asthma model mice. Biol Pharm Bull. 2005;28:829–833. doi: 10.1248/bpb.28.829. [DOI] [PubMed] [Google Scholar]
- 9.Fu KL, Lu J, Jiao Y. Investigation on TCM Syndromes of 99 Cases of Chronic Obstructive Pulmonary Diseases. Journal of Traditional Chinese Medicine. 2007;48:923–926. [Google Scholar]
- 10.Gagnier JJ, DeMelo J, Boon H, Rochon P, Bombardier C. Quality of reporting of randomized controlled trials of herbal medicine interventions. Am J Med. 2006;119:801.e1–801.e11. doi: 10.1016/j.amjmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Gold members, author. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. [Feb 3, 2013]. On document at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
- 12.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2013. [Feb 3, 2013]. Online document at: http://www.cochrane-handbook.org.
- 13.Hong M, Wang XZ, Wang L, Hua YQ, Wen HM, Duan JA. Screening of immunomodulatory components in Yu-ping-feng-san using splenocyte binding and HPLC. J Pharm Biomed Anal. 2011;54:87–93. doi: 10.1016/j.jpba.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Huang DH, Wu L, He DP, Lin L. Clinical observation of tranquilization period of chronic obstructive pulmonary disease treated by integration of traditional Chinese medicine and Western medicine. J Fourth Mil Med Univ. 2005;26:1611–1613. [Google Scholar]
- 15.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 16.Li DJ, Yuan HQ, Liu HX, Zhu Y. Effects of Jiawei yupingfeng san on pulmonary ventilation function of patients with stable chronic obstructive pulmonary disease. Journal of Emergency in Traditional Chinese Medicine. 2010;19:1679–1680. [Google Scholar]
- 17.Liao YL, An W, Zhang W. The efficacy of Yupingfeng san as an adjunctive therapy for stable chronic obstructive pulmonary disease. Herald of Medicine. 2011;30:63–66. [Google Scholar]
- 18.Liu L, Gao SL, Niu FQ. Application analysis of Jiawei yupingfeng san for the treatment of stable chronic obstructive pulmonary disease. Guide of China Medicine. 2012;10:574–576. [Google Scholar]
- 19.Makino T, Ito Y, Sasaki SY, Fujimura Y, Kano Y. Preventive and curative effects of Gyokuheifu-san, a formula of traditional Chinese medicine, on allergic rhinitis induced with Japanese cedar pollens in guinea pig. Biol Pharm Bull. 2004;27:554–558. doi: 10.1248/bpb.27.554. [DOI] [PubMed] [Google Scholar]
- 20.Makino T, Sasaki SY, Ito Y, Kano Y. Pharmacological properties of traditional medicine (XXX): effects of Gyokuheifusan on murine antigen-specific antibody production. Biol Pharm Bull. 2005;28:110–113. doi: 10.1248/bpb.28.110. [DOI] [PubMed] [Google Scholar]
- 21.Makino T. Pharmacological properties of Gyokuheifusan, a traditional Kampo medicinal formula. Yakugaku Zasshi. 2005;125:349–354. doi: 10.1248/yakushi.125.349. [DOI] [PubMed] [Google Scholar]
- 22.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schünemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 23.Scales DC, Rubenfeld GD. Estimating sample size in critical care clinical trials. J Crit Care. 2005;20:6–11. doi: 10.1016/j.jcrc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D CONSORT Group, author. ONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedgwick P. Meta-analyses: how to read a funnel plot. BMJ. 2013;346:f1342. doi: 10.1136/bmj.h4718. [DOI] [PubMed] [Google Scholar]
- 26.Shah KN, Panchal DJ, Vyas BM, Patel VJ. Use of placebo: knowledge, attitude and practice among medical practitioners. Indian J Med Sci. 2009;63:472–473. [PubMed] [Google Scholar]
- 27.Song J, Li J, Zheng SR, Jin Y, Huang Y. Anti-inflammatory and immunoregulatory effects of Yupingfeng powder on chronic bronchitis rats. Chin J Integr Med. 2013;19:353–359. doi: 10.1007/s11655-013-1442-6. [DOI] [PubMed] [Google Scholar]
- 28.Sorkness RL. CAM and respiratory disease. Nutr Clin Pract. 2009;24:609–615. doi: 10.1177/0884533609342438. [DOI] [PubMed] [Google Scholar]
- 29.Wang YR, Shi YL, Wang XY. Efficacy of Yupingfeng san for improving lung function and quality of life for patients with stable chronic obstructive pulmonary disease. Evaluation and Analysis of Drug-Use in Hospitals of China. 2012;12:823–825. [Google Scholar]
- 30.Xu J. Clinical observation of Yupingfeng granule for the treatment of chronic obstructive pulmonary disease. Journal of Emergency in Traditional Chinese Medicine. 2010;19:381, 404. [Google Scholar]
- 31.Xu XH. Study on the clinical efficiency of Yushenyupingfengtang for the treatment of chronic obstructive pulmonary disease. Chinese Journal of Hospital Pharmacy. 2010;30:2102–2105. [Google Scholar]
- 32.Yang YF, Ross J. Chinese Herbal Formulas. Edinburgh: Churchill Livingstone; 2010. Deficiency syndrome and formula composition; pp. 129–190. [Google Scholar]
- 33.Zhang RZ, Zheng SJ, Yan GZ. Clinical research of modified Yupingfeng san on patients with moderate chronic obstructive pulmonary disease. China Practical Medical. 2011;6:118–119. [Google Scholar]
- 34.Zhao BQ, Mai MQ. linical observation of modified Yupingfeng san for the treatment of chronic obstructive pulmonary disease. Journal of Emergency in Traditional Chinese Medicine. 2009;18:6–7. [Google Scholar]
- 35.Zhen XY. Guiding principle of clinical research on new drugs of traditional Chinese medicine (trial implementation) Beijing: Chinese Medical Science and Technology Press; 2002. pp. 54–58. [Google Scholar]