Abstract
A fluorine-labelled zinc(II)-dipicolylamine coordination complex reports the presence of phosphate anions in aqueous solution, especially pyrophosphate and ADP, and is used to monitor the enzymatic hydrolysis of ATP.
19F NMR has many attractive features that encourage its use in biomedicine.1 The 19F nucleus has a natural abundance of 100% and signal sensitivity of 83% relative to 1H NMR. 19F NMR spectra of small molecule samples at submillimolar concentrations can be acquired with high signal to noise. Furthermore, recent breakthroughs in NMR signal enhancement, using techniques that exploit dynamic nuclear polarization, allow detection of 19F signals from samples at submicromolar concentrations.2 The lack of endogenous fluorine in most biomedical samples eliminates background interference problems, and the wide dispersion of 19F chemical shifts diminishes the chance of complications due to overlapping signals. It is not surprising that 19F NMR is an emerging strategy for magnetic resonance imaging and is often considered for development into drug screening assays.3
A requirement for essentially all of these 19F NMR techniques is development of fluorine-labelled reporter molecules. Since many drug structures contain fluorine atoms, it is logical to directly study fluorine-labelled fragments or drug candidates using 19F NMR screening methods that monitor a pharmaceutically relevant protein binding process.4 In other cases, the 19F label is incorporated within the structure of an enzyme substrate and the change in chemical shift upon enzyme action is detected as an assay output signal.5 Fluorine-labelled enzyme substrates have also been incorporated into new methods for 19F MRI. The results of enzyme action alter the substrate structure and produce either a change in signal chemical shift or relaxation time.6 The central concept that is described here is a 19F NMR reporter with ability to undergo a change in chemical shift upon reversible association with a molecular target. The few literature examples of this strategy include 19F labelled reporters that respond reversibly to temperature,7 pH,8 metal cations,9 diol-containing molecules,10 and protease enzymes.11
Herein, we describe the recognition properties of compound 1 (Figure 1) as the first example of a 19F NMR reporter that can detect the presence of biologically relevant phosphate anions.12 Compound 1 is a zinc(II)-dipicolylamine (ZnDPA) coordination complex.13 The organic scaffold is a phenol derivative with two ortho-substituted 2,2'-dipicolylamine groups and a fluorine atom attached to the para carbon. The oxyanion recognition properties of this class of ZnDPA complexes have been studied quite extensively, and strong association is typically observed with polyanionic phosphate esters.14 Most notably, optically active versions of these compounds with chromophores appended to the para carbon of the phenol scaffold have been observed to undergo red-shifts in absorption maxima.14b, 14d, 14g A logical explanation for this effect is that phosphate coordination to the zinc cations pushes electron density onto the para carbon and into the attached chromophore. If true, we reasoned that a fluorine label on the para carbon would exhibit a predictable change in chemical shift upon phosphate binding. Tentative support for this idea was a reported difference in 19F chemical shifts for the zinc and copper salts of compound 1 (measured in the absence of coordinating anions).15
Fig 1.
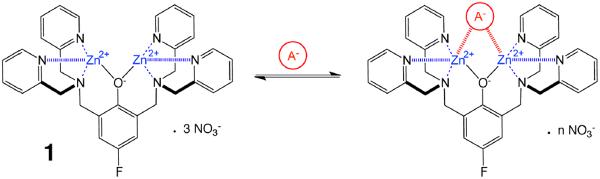
Reversible association of 19F NMR reporter 1 with a bidentate anion.
Structural evidence for our design concept was gained by elucidating the solid-state complex of reporter 1 bound to dihydrogen phosphate. We discovered that mixing compound 1 with sodium phosphate in water produced single crystals that were amenable to analysis by X-ray diffraction.‡ Refinement of the data produced the molecular structure shown in Figure 2. The general coordination features and bond lengths agree with related literature structures.16 As expected, the bidentate dihydrogen phosphate dianion bridges the two zinc cations in 1. One zinc cation adopts a five-coordinate geometry and the other is six-coordinate with a water molecule occupying the additional coordination site. Related crystal structures in the literature with poylanionic phosphates such as pyrophosphate (PPi) or ADP show tetradentate coordination with four phosphate oxygens coordinated to the two zinc cations.14b, 14c, 14f Together, the X-ray structures suggest that tetradentate coordination of poylanionic phosphates to compound 1 will push electron density onto the para carbon and produce an upfield change in 19F chemical shift for the attached fluorine.
Fig 2.
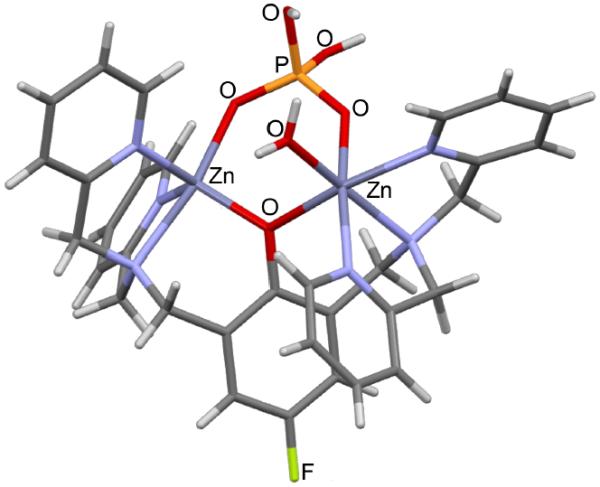
X-ray crystal structure of 1(H2PO4)(NO3)2•H2O. The structure omits the two NO3− anions for clarity.
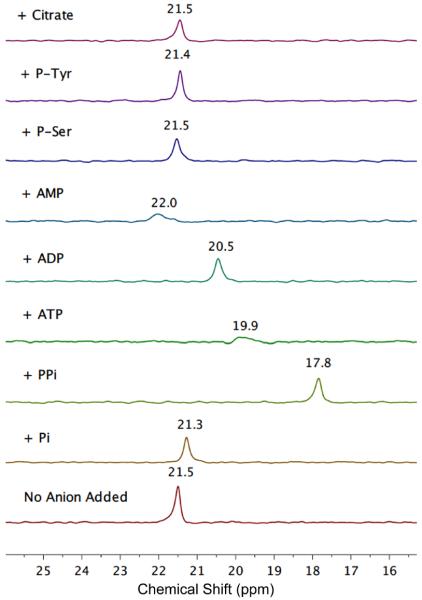
19F NMR titration studies added incremental amounts of oxyanions to separate samples of compound 5 1 (0.40 mM in 50 mM HEPES buffer, pH=7.2).¶ Shown in Figure 3 are partial F NMR spectra of 1 in the absence and presence of one molar equivalent of different oxyanions. The F chemical shift for compound 1 was observed at 21.5 ppm (internal KBF4 as reference) and there was no detectable signal change produced by addition of P-Ser, P-Tyr, or citrate. Significant changes in chemical shift were produced by addition of PPi, ATP, ADP, phosphate (Pi), and AMP. The anions with high amounts of negative charge produced relatively large upfield changes in chemical shift. The slightly downfield effect produced by AMP is attributed to anisotropic shielding by the proximal adenosine ring. In the case of ATP binding, a broad F signal was observed, even when 1 was saturated with ATP, indicating an intramolecular process that exchanges the bound ATP between different phosphate zinc coordination geometries. As expected, binding constants with these polyanionic phosphates were too strong (Ka > 104 M−1) for accurate determination by NMR.§ A further complication preventing NMR measurements of association was the intermolecular exchange broadening that occurred when the ratio of anion to 1 was sub-stoichiometric (see ESI). Therefore we measured relative anion binding affinities by conducting competition experiments that mixed reporter 1 with different ratios of competing anions. Shown in Figure 4 are selected spectra for samples that contained equal amounts of two competing anions. Analysis of all the data indicates that 1 has a relative affinity order of PPi > ADP ≈ ATP > Pi.
Fig 3.
Partial 19F NMR spectra (564 MHz) of reporter 1 (0.40 mM in HEPES buffer, pH 7.2) with no anion added (bottom spectrum) and in the presence of one molar equivalent of the following anions as their sodium salts; phosphate (Pi); pyrophosphate (PPi); ATP; ADP; AMP; o-phospho-L-serine (P-Ser); o-phospho-L-tyrosine (P-Tyr); citrate.
Fig 4.
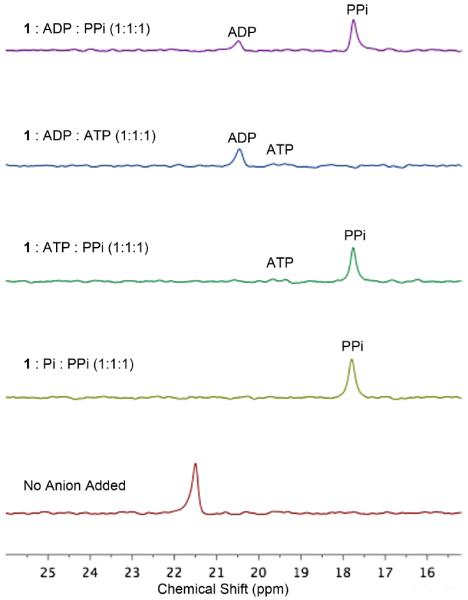
Partial 19F NMR spectra (564 MHz) of reporter 1 mixed with two competing anions (as their sodium salts). Each of the three components was 0.40 mM in HEPES buffer, pH 7.2.
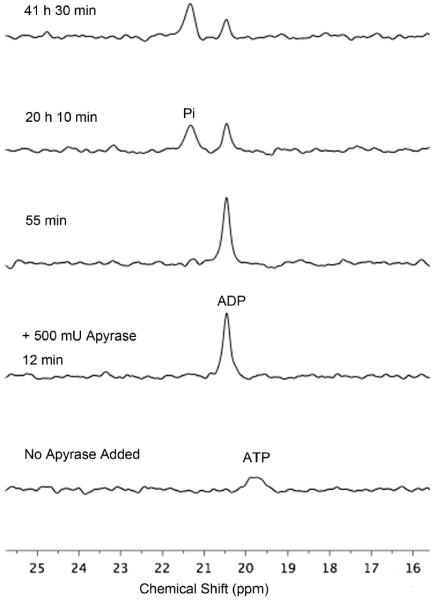
To demonstrate the potential utility of reporter 1, we used it to track an enzymatic reaction; namely, the hydrolysis of ATP catalysed by commercially available apyrase.17 The F spectra of reporter molecule 1 in Figure 5 reflect sequential conversion of ATP into ADP followed by further hydrolysis and production of Pi. The spectra show clearly that the first step in the process (hydrolysis of ATP) is complete after 12 minutes, whereas the second step (hydrolysis of ADP) is much slower and takes well over 41 hours. The high ATPase/ADPase ratio was confirmed by independent experiments that started with a sample of pure ADP (see ESI) and closely matched the vendor's certification of relative enzyme activities. These enzyme hydrolysis experiments highlight the power of this NMR method to report kinetic information for sequential steps in the same assay sample. In contrast, fluorescent probes that can only report single steps, such as ATP consumption through emission intensity changes, are unable to provide information about any subsequent chemistry.17 In principle, it should be possible to use compound 1 to monitor other reactions that consume ATP such as kinase catalyzed phosphorylation processes. Compound 1 may also be useful in cell imaging studies of polyphosphate anions.
Fig 5.
Partial 19F NMR spectra (564 MHz) of a mixture of reporter 1 and ATP (both 0.40 mM in HEPES buffer, pH 7.2) before addition of apyrase (bottom spectrum) and at increasing 5 time points thereafter.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM059078).
Footnotes
Electronic Supplementary Information (ESI) available: Synthesis, X-ray, and NMR data. See DOI: 10.1039/b000000x/
A detailed description of the X-ray structure is provided in the ESI. The crystallographic data is also provided in CCDC 929896, which can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax (+44) 1223-336-033; or deposit@ccdc.cam.uk).
Typical 19F NMR samples contained 0.40 mM of 1 in 0.5 mL of 50 mM HEPES buffer at 22 °C, pH=7.2 with an external lock sample containing D2O. The internal reference was KBF4 (−150.4 ppm relative to CFCl3) and T1 for the 19F signal of free 1 was measured to be 0.59±0.01 second at 22 °C (operating frequency was 564 MHz). A typical acquisition collected 1000 scans using a 30° pulse and 0.10 second relaxation delay.
A close structural analogue of 1 is reported to bind PPi and Pi with association constants of 6.7 × 106 M−1 and 1.1 × 105 M−1, respectively.14h
Notes and references
- 1.Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Zeng H, Ruedisser S, Gossert AD, Hilty C. J. Am. Chem. Soc. 2012;134:17448–17451. doi: 10.1021/ja308437h. [DOI] [PubMed] [Google Scholar]
- 3.(a) Yu JX, Kodibagkar VD, Cui W, Mason RP. Curr. Med. Chem. 2005;12:819–848. doi: 10.2174/0929867053507342. [DOI] [PubMed] [Google Scholar]; (b) Knight JC, Edwards PG, Paisey SJ. RSC Adv. 2011;1:1415–1425. [Google Scholar]; (c) Dalvit C. Prog. Nucl. Mag. Res. Sp. 2007;51:243–271. [Google Scholar]
- 4.(a) Papeo G, Giordano P, Brasca MG, Buzzo F, Caronni D, Ciprandi F, Mongelli N, Veronesi M, Vulpetti A, Dalvit C. J. Am. Chem. Soc. 2007;129:5665–5672. doi: 10.1021/ja069128s. [DOI] [PubMed] [Google Scholar]; (b) Peng JW. J. Magn. Reson. 2001;153:32–47. doi: 10.1006/jmre.2001.2422. [DOI] [PubMed] [Google Scholar]
- 5.Stockman BJ. J. Am. Chem. Soc. 2008;130:5870–5871. doi: 10.1021/ja801588u. [DOI] [PubMed] [Google Scholar]
- 6.(a) Mizukami S. Chem. Pharm. Bull. 2011;59:1435–1446. doi: 10.1248/cpb.59.1435. [DOI] [PubMed] [Google Scholar]; (b) Matsushita H, Mizukami S, Mori Y, Sugihara F, Shirakawa M, Yoshioka Y, Kikuchi K. ChemBiochem. 2012;13:1579–1583. doi: 10.1002/cbic.201200331. [DOI] [PubMed] [Google Scholar]
- 7.Vidrine DW, Peterson PE. Anal. Chem. 1978;50:298–303. [Google Scholar]
- 8.Deutsch CJ, Taylor JS. Biophys. J. 1989;55:799–804. doi: 10.1016/S0006-3495(89)82879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy LA, Gabel SA, London RE. Magn. Reson. Chem. 1996;34:440–446. [Google Scholar]
- 10.London RE, Gabel SA. J. Am. Chem. Soc. 1994;116:2562–2569. [Google Scholar]
- 11.London RE, Gabel SA. J. Am. Chem. Soc. 1994;116:2570–2575. [Google Scholar]
- 12.For 19F NMR reporters of other oxyanions, see: Plenio H, Diodone R. Z. Naturforsch. B. 1995;50:1075–1078. Harvey P, Chalmers KH, De Luca E, Mishra A, Parker D. Chem. Eur. J. 2012;18:8748–8757. doi: 10.1002/chem.201200737.
- 13.(a) Sakamoto T, Ojida A, Hamachi I. Chem. Commun. 2009:141–152. doi: 10.1039/b812374h. [DOI] [PubMed] [Google Scholar]; (b) Kruppa M, Konig B. Chem. Rev. 2006;106:3520–3560. doi: 10.1021/cr010206y. [DOI] [PubMed] [Google Scholar]; (c) O'Neil EJ, Smith BD. Coord. Chem. Rev. 2006;250:3068–3080. [Google Scholar]; (d) Kim SK, Lee DH, Hong JI, Yoon J. Acc. Chem. Res. 2009;42:23–31. doi: 10.1021/ar800003f. [DOI] [PubMed] [Google Scholar]; (e) Zhou Y, Xu Z, Yoon J. Chem. Soc. Rev. 2011;40:2222–2235. doi: 10.1039/c0cs00169d. [DOI] [PubMed] [Google Scholar]; (f) Ngo HT, Liu X, Jolliffe KA. Chem. Soc. Rev. 2012;41:4928–4965. doi: 10.1039/c2cs35087d. [DOI] [PubMed] [Google Scholar]; (g) Drewry JA, Burger S, Mazouchi A, Duodu E, Ayers P, Gradinaru CC, Gunning PT. Med. Chem. Comm. 2012;3:763–770. doi: 10.1021/ic3008393. [DOI] [PubMed] [Google Scholar]
- 14.Selected examples: Han MS, Kim DH. Angew. Chem. Int. Ed. 2002;41:3809–3811. doi: 10.1002/1521-3773(20021018)41:20<3809::AID-ANIE3809>3.0.CO;2-N. Lee DH, Im JH, Son SU, Chung YK, Hong JI. J. Am. Chem. Soc. 2003;125:7752–7753. doi: 10.1021/ja034689u. Lee JH, Park J, Lah MS, Chin A, Hong JI. Org. Lett. 2007;9:3729–3731. doi: 10.1021/ol071306e. Lee DH, Kim SY, Hong JI. Angew. Chem. Int. Ed. 2004;43:4777–4780. doi: 10.1002/anie.200453914. Honda K, Fujishima SH, Ojida A, Hamachi I. ChemBiochem. 2007;8:1370–1372. doi: 10.1002/cbic.200700146. Huang F, Cheng C, Feng G. J. Org. Chem. 2012;77:11405–11408. doi: 10.1021/jo302271t. Pathberiya LG, Barlow N, Nguyen T, Graham B, Tuck KL. Tetrahedron. 2012;68:9435–9439. Hanshaw RG, Hilkert SM, Jiang H, Smith BD. Tetrahedron Lett. 2004;45:8721–8724.
- 15.Torelli S, Belle C, Gautier-Luneau I, Hamman S, Pierre JL. Inorg. Chim. Acta. 2002;333:144–147. [Google Scholar]
- 16.Jiang ONEJ,H, Gassensmith JJ, Smith BD. Supramol. Chem. 2013 doi: 10.1080/10610278.2013.776170. [Google Scholar]
- 17.(a) Xu Z, Singh NJ, Lim J, Pan J, Kim HN, Park S, Kim KS, Yoon J. J. Am. Chem. Soc. 2009;131:15528–15533. doi: 10.1021/ja906855a. [DOI] [PubMed] [Google Scholar]; (b) Arman SV, Czarnik AW. Supramol. Chem. 1993;1:99–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.