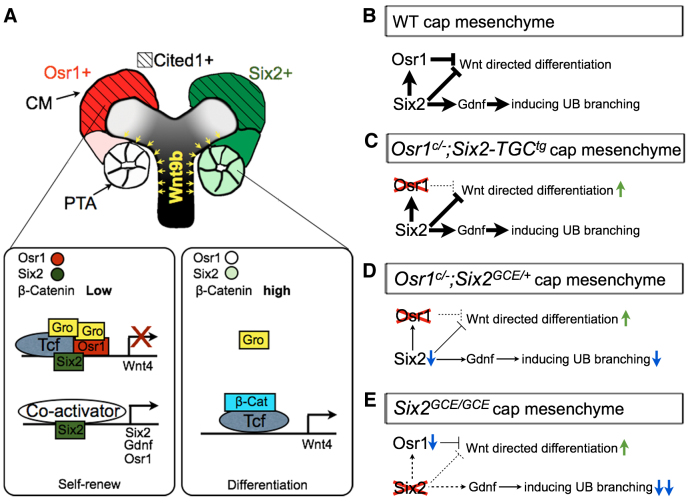
Fig. 11.
A model depicting the molecular mechanism involving Osr1, Six2 and Wnt9b/β-catenin signaling in the regulation of nephron progenitor renewal and differentiation. (A,B) In wild-type kidney, Osr1 and Six2 interact and form a strong repressor complex with TCF and Groucho/Tle proteins to prevent the activation of Wnt/β-catenin target genes such as Wnt4 in the CM. Six2 also acts independently of Osr1 to activate expression of many CM genes, including itself, Gdnf and Osr1. The CM cells in the ‘armpit’ of new UB branches receive the highest level of Wnt9b signaling, which converts TCF from repressor to activator and activates expression of differentiation genes, including Wnt4. (C) In Osr1c/-;Six2-TGCtg mutant embryos, Osr1 deficiency in the CM causes premature differentiation but Six2 is still expressed at high levels and maintains expression of many CM-specific genes, including Gdnf. (D) In Osr1c/-;Six2GCE/+ mutant embryos, loss of Osr1 and significant downregulation of Six2 cause earlier onset of premature nephrogenic differentiation and reduced UB branching than in Osr1c/-;Six2-TGCtg mutant embryos. (E) In Six2GCE/GCE mutant embryos, the complete lack of Six2 causes rapid loss of both Gdnf and Osr1, resulting in rapid depletion of CM cells as well as lack of UB branching.