FIG 1.
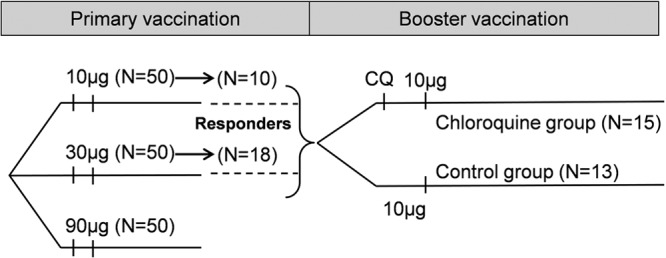
Study design. Study participants received two doses of the F4/AS01B investigational vaccine (10, 30, or 90 μg of F4 recombinant protein per dose) in a previous study (primary vaccination; study NCT00434512) and a third dose (10 μg of F4 recombinant protein per dose) in the current study (booster vaccination). Responders, participants with a frequency of antigen-specific CD4+ T cells of >0.03% at 44 days after the first dose of the F4/AS01B vaccine in the previous study; N, number of participants; CQ, chloroquine.
