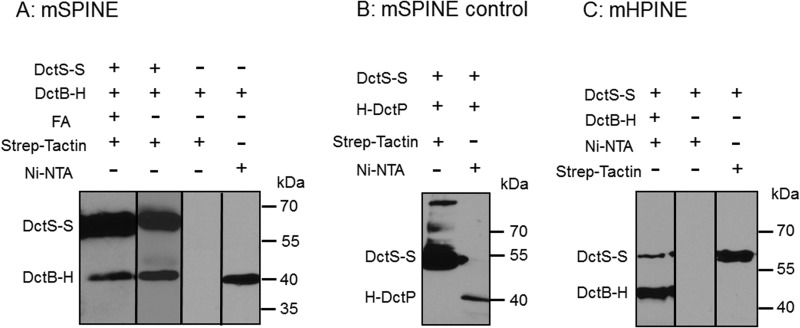
FIG 1.
In vivo binding of DctB-His6 (DctB-H) to DctS-Strep (DctS-S) and copurification using a Strep-Tactin column (mSPINE) (A) and a Ni-NTA column (mHPINE) (C). (A) The two proteins were coexpressed in E. coli C43(DE3). Formaldehyde (FA) was added at an OD578 of 1.3 to 1.6 where indicated. The membrane fraction was isolated and solubilized with 2% (wt/vol) Empigen. DctS-S was isolated from the membrane fraction using a Strep-Tactin column, and 20 μl of the eluate was subjected to SDS gel electrophoresis and Western blotting. As a control, DctB-H was expressed without DctS-S. (B) For testing the specificity of the mSPINE assay, the two noninteracting proteins DctS-S and His-DctP from Aromatoleum aromaticum were coexpressed in E. coli C43(DE3). At an OD578 of 1.3 to 1.6, the cells were incubated with formaldehyde. The membrane fraction was solubilized as described above, and DctS-S was isolated using a Strep-Tactin column. (C) For the mHPINE assay, DctB-H was isolated from the solubilized membrane fraction using a Ni-NTA column. As a control, DctS-S was expressed without DctB-H.