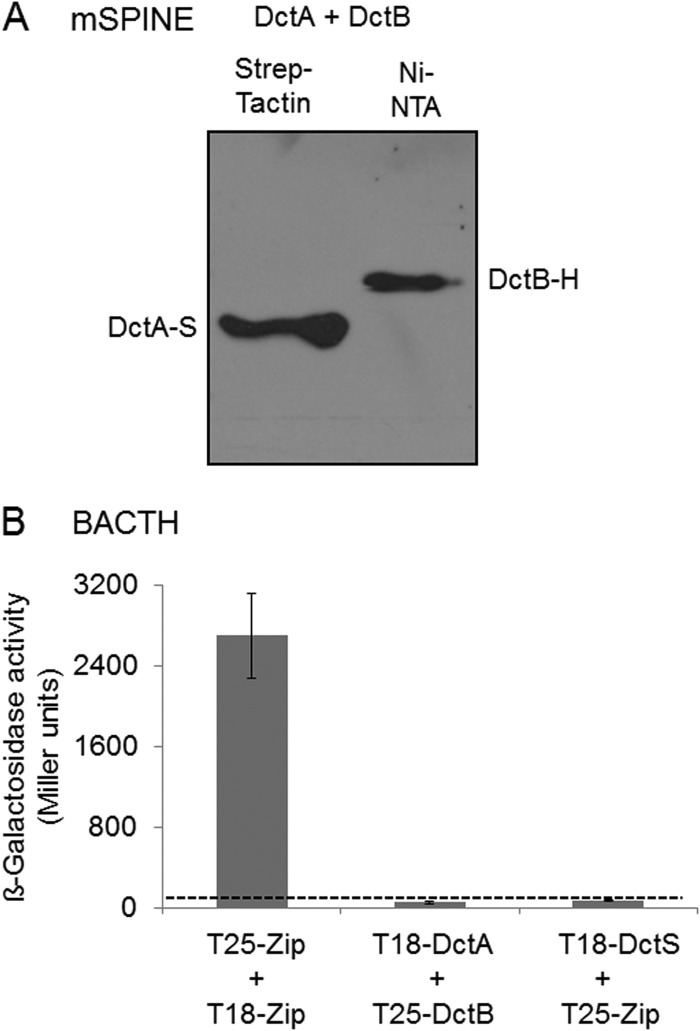
FIG 3.
Lack of interaction of DctA with the binding protein DctB: studies using the mSPINE assay followed by Western blotting (A) and the BACTH assay (B). For the mSPINE assay, DctA-Strep (DctA-S) and DctB-His (DctB-H) were coexpressed in E. coli C43(DE3) and the membranes were solubilized using 2% (wt/vol) Empigen BB detergent. DctA-S was isolated from the solubilized membrane fraction using a Strep-Tactin column. For the BACTH assays, E. coli BTH101 was cotransformed with plasmid pairs. In each pair, one plasmid encoded a T25 fusion and the other encoded a T18 fusion: T25-DctS (pMW1697) and T25-DctB (pMW1705) were the T25 fusions, and DctR-T18 (pMW1699), T18-DctA (pMW1704), and T18-DctS (pMW1696) were the T18 fusions. The β-galactosidase activity was determined after growth to an OD578 of 0.5 to 0.7. The strain expressing the pair of leucine zipper proteins was used as a positive control for two interacting cytosolic proteins. The background activity obtained for noninteracting proteins like T18-DctS plus T25-Zip was below 80 Miller units (dashed line). All results are the averages of the measurements of at least four biological replicates. Error bars represent the standard deviations.