Abstract
A voluntary, cost-free external quality assessment (EQA) program established by the U.S. Centers for Disease Control and Prevention (CDC) was implemented to primarily monitor the performance of laboratories conducting HIV Early Infant Diagnosis (EID) from dried blood spots (DBS) in low- to middle-income countries since 2006. Ten blind DBS proficiency test (PT) specimens and 100 known HIV-positive and -negative DBS specimens (to be used as internal controls) were shipped triannually to participating laboratories with reports for the PT specimens due within 30 days. The participant's results and a summary of the performance of all participating laboratories and each diagnostic method were provided after each test cycle. Enrollment in the CDC PT program expanded progressively from 17 laboratories from 11 countries in 2006 to include 136 laboratories from 41 countries at the end of 2012. Despite external pressures to test and treat more children while expanding EID programs, mean PT test scores significantly improved over time as demonstrated by the upward trend from mid-2006 to the end of 2012 (P = 0.001) and the increase in the percentage of laboratories scoring 100% (P = 0.003). The mean test scores plateaued over the past 10 testing cycles, ranging between 98.2% and 99.7%, and discordant test results still occur but at a rate of no higher than 2.6%. Analysis of these test results suggests a positive impact of proficiency testing on the testing performance of the participating laboratories, and a continuous training program and proficiency testing participation may translate into laboratories improving their testing accuracy.
INTRODUCTION
Worldwide, children account for nearly 1 in 5 of all HIV-related deaths and 1 in 6 of new HIV infections each year. More than 90% of the annual 400,000 pediatric HIV infections globally are acquired vertically through mother-to-child transmission (1), and most of them occur in sub-Saharan Africa. Early infant diagnosis (EID) can remarkably impact an infant's survival. If the exposed child receives early diagnosis and is referred to and initiates proper treatment, then early mortality and morbidity can be greatly reduced (2, 3).
Routine HIV diagnostic testing such as highly accurate rapid tests and enzyme immunoassays that are designed for older children and adults do not meet the needs of infants and young children (<18 months of age) because infants of HIV-positive mothers acquire antibody transplacentally and young infants test antibody positive regardless of their HIV infection status, which can persist in the child for up to 18 months after birth. However, routine testing for HIV antibodies in infants can be used to screen for HIV exposure before the age of 18 months and is highly informative after 9 months of age in resource-limited settings (RLS) when it is an integral part of national testing algorithms (4). Virologic assays, including HIV-1 DNA or RNA assays, represent the gold standard for detecting HIV-1 DNA/RNA in human whole blood and presumptively diagnosing HIV infection in infants and children younger than 18 months of age. With such testing, the diagnosis of HIV-1 can be established within the first several weeks of life. The World Health Organization (WHO) strongly recommends that virologic testing be performed at 4 to 6 weeks of age or as early as possible thereafter and at 6 weeks after weaning (4, 5). In developed countries, HIV DNA or RNA PCR is repeated for confirmation. However, in RLS, despite the WHO recommendation to collect a second dried blood spot (DBS) for confirmatory testing, often only a single HIV DNA PCR is performed due to the loss to follow-up and long testing turnaround time (TAT). This reinforces the need for ensuring the quality of PCR testing.
For settings that lack adequate infrastructure for processing whole blood and cold-chain transportation, the DBS offers many advantages. The DBS is a reliable source of analyte, which increases access to testing by its ease of collection via heel prick or finger stick, the lack of need for cold-chain transportation, ease of transport from remote areas to more centralized locations for testing, elimination of biological hazard concerns, and stability for long periods of time at ambient temperature (6–11). Collection of the DBS has facilitated the detection of HIV-1 in infants as early as at birth and at 4 to 6 weeks after birth (12–17).
The surge in funding from the U.S. President's Global Health Initiative and the President's Emergency Plan For AIDS Relief (PEPFAR), The Global Fund to Fight AIDS, Tuberculosis and Malaria, World Bank, Clinton Health Access Initiative, and other major donors and initiatives has allowed the rapid expansion of EID testing to areas in sub-Saharan Africa and other regions of the world previously unable to perform EID testing. To ensure the accuracy of EID of HIV, external quality assessment (EQA) is essential. A major component of a laboratory's quality assurance program is proficiency testing (PT). PT has been shown to improve the quality of testing for various human diseases and analytes (18–29). In response to the need for a PT program, the CDC initiated a voluntary HIV-1 DNA DBS PT Program in 2006 that is primarily designed for laboratories receiving PEPFAR funds for HIV-related activities in RLS but that also includes non-PEPFAR-funded facilities.
MATERIALS AND METHODS
(i) Participation in the EQA Program.
Laboratory facilities conducting HIV-1 EID using DBS with PEPFAR support were informed of the PT program and encouraged to participate. Originally, 15 countries participated as soon as they developed DNA PCR EID testing capacity. The identities of the participating laboratories were kept confidential. As the PT Program expanded, all PEPFAR-supported and other non-PEPFAR-supported countries were encouraged to enroll and participate.
(ii) Preparation of HIV-1 DNA DBS PT Panel.
The DBS panel consisted of 10 blind DBS specimens: a combination of HIV-1-positive DBSs and HIV-1-negative DBSs. These positive DBS samples were prepared by spiking negative EDTA–anticoagulated whole-blood samples (Tennessee Blood Services, Memphis, TN) with known numbers of 8E5 cells, which contain a single integrated defective copy of the HIV-1 proviral DNA per cell (30). Freshly grown 8E5 cells were counted using a hemocytometer and diluted to three concentrations (10,000, 5,000, or 1,250 cells/ml) in whole blood. DBS cards were prepared by transferring 100 μl of HIV-negative whole blood or HIV-1-spiked whole blood to Whatman 903 filter cards (Whatman, Piscataway, NJ). The HIV-positive and HIV-negative DBS cards were prepared in separate processing rooms, dried overnight at room temperature in racks and wrapped in glassine paper, and stored in liquid-tight plastic sealable storage bags (10 per bag) (Fisher, Waltham, MA) with desiccant sachets (Multisorb Technologies, Buffalo, NY) and a humidity indicator card (Multisorb Technologies, Buffalo, NY) at −20°C.
(a) Coding of PT panel.
Every testing cycle included a DBS panel that contained 10 blind DBSs with at least five HIV-positive specimens with different HIV copy numbers. The coding for each panel was randomized and thus different from run to run.
(b) Preparation of PT panel: 6-mm-diameter disc.
Two PT panels were prepared, a 6-mm-diameter disc and a whole-card panel. Most facilities performing HIV-1 EID used a Roche Amplicor HIV-1 DNA Test, v1.5 (Roche Diagnostics, Indianapolis, IN), with a modification in the nucleic acid extraction step designed for a 6-mm-diameter disc excised from a DBS card. In the Atlanta CDC facility, the 6-mm-diameter discs were excised from the validated DBS cards using a BSD-600 Duet instrument (BSD Robotics, Brisbane, Australia) and placed inside 2-ml cryovial tubes labeled with the appropriate panel and specimen identifier. Each PT package included the following 4 items: (i) a liquid-tight specimen storage bag with desiccant sachets and humidity indicator card and 10 2-ml cryovial tubes each containing a DBS disc (the blind panel), (ii) 100 labeled HIV-positive and HIV-negative DBSs to be used as internal quality control specimens for all subsequent DNA PCR testing, (iii) testing and reporting instructions, and (iv) a test reporting form.
(c) Preparation of PT panel: 13-mm-diameter disc.
Facilities using a Roche Cobas Ampliprep/TaqMan system (Roche Diagnostics, Indianapolis, IN) for extraction and detection or a Roche MagNA Pure LightCycler (LC) system (Roche Diagnostics, Indianapolis, IN) for nucleic acid extraction requested a full 100-μl DBS (13-mm-diameter disc) specimen for testing according to their standard operating procedures. The DBSs were excised using scissors and placed inside appropriately labeled 15-ml Falcon tubes (Becton, Dickinson Biosciences, San Jose, CA). The PT package included the same 4 items as described above except for the inclusion of 13-mm-diameter discs.
(iii) Panel validation of DBS cards prior to the testing cycle.
The validity and integrity of the DBS PT were tested before panel assembly and again prior to shipment. Initially, the DBS PT cards were validated by randomly selecting one DBS each from 10 randomly selected DBS cards and excising either a 6-mm-diameter or 13-mm-diameter disc from the DBS. Each disc was tested at CDC using a Roche Amplicor HIV-1 DNA Test, v1.5 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Subsequently, five cards from the beginning, middle, and end of each DBS production were validated by using a Roche Amplicor HIV-1 DNA Test, v1.5, and Roche Cobas Ampliprep/TaqMan. Validation results were compared within and between batches, and the values were consistent.
(iv) Shipping.
The PT panels and internal controls were shipped at ambient temperature to the participant facilities via air cargo. CDC, WHO, and International Air Transport Association (IATA) DBS specimen shipping guidelines were followed (31–33).
(v) Results reporting and analysis.
Participating laboratories were expected to report the results of the PT testing (3 per year) within 30 days from the receipt of the shipment. Laboratories were evaluated on the concordance of their results with expected results previously validated prior to shipment by CDC. Test scores (number of correct results of 10 tests per test cycle) and peer comparison results were returned to the participants via email within 4 weeks of receipt. Laboratories that scored 80% or lower were contacted by email or phone to discuss potential sources of error.
(vi) Statistical analysis.
Normally distributed data were expressed as means and standard deviations, and data from non-normally distributed variables were expressed as medians and interquartile ranges. Linear regression was used to examine the relationship between mean score and EID laboratories per country and number of testing cycles participated in. Linear regression for trend was used to analyze for changes in mean score and percentages of laboratories scoring 100% over time. Statistical calculations were performed using SPSS (Statistical Package for the Social Sciences) software (version 21.0; IBM, Armonk, NY), with significance defined as P < 0.05.
RESULTS
Since implementation of the CDC PT program, 20 test cycles have been conducted, including 2 in 2006 and 3 each year in 2007 to 2012. The number of participating laboratories steadily increased from 17 in 11 PEPFAR-supported countries in 2006 to 136 in 38 PEPFAR-supported and 5 in 3 non-PEPFAR-supported countries in 2012. The numbers of participating laboratories were 23, 45, 68, 81, 99, 120, and 136 at the end of 2006, 2007, 2008, 2009, 2010, 2011, and 2012, respectively. The distribution of participating facilities by the end of 2012 was as follows: 105 laboratories from 24 sub-Saharan African countries, 18 laboratories from 7 Asian countries, 6 laboratories from the Caribbean, 2 laboratories each from North and South America, and 3 laboratories from Oceania.
A majority (91.8%) of the participating laboratories (range, 76.5% to 100%; median, 93.6%) returned the PT testing results electronically to CDC Atlanta after each testing cycle. As a measure of performance, the mean scores were determined for each testing cycle (Fig. 1). The mean range was 93.5% to 99.7%. The lowest mean score (93.5%) was observed in the first PT cycle, and the highest mean scores of 99.6% and 99.7% were recorded in the last PT cycle of 2009 and first of 2010. The mean scores increased significantly over time for the first 12 testing cycles (R2 = 0.60; P = 0.003), peaking at the 12th testing cycle. Following the 12th testing cycle, the mean scores remained in a very tight range of 98.2% to 99.2%.
FIG 1.
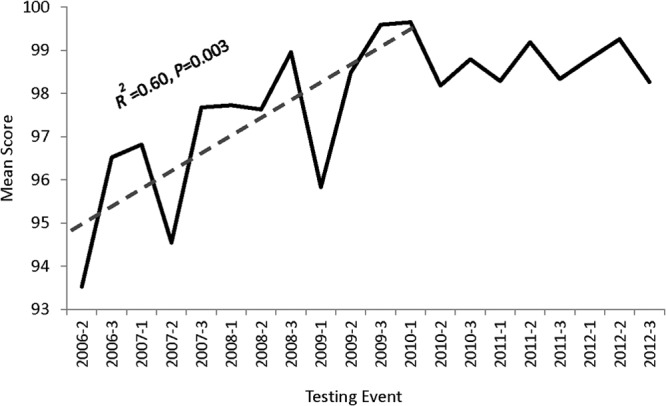
Mean score over the course of 20 testing cycles. A linear regression for trend was used to analyze for changes in mean score over time from testing cycle 2 in 2006 (2006-2) to 2010-1.
In 2006, the Roche Amplicor HIV-1 DNA Test, v1.5, was recommended for EID by the WHO and CDC (34), and it was the only assay reported to be used by participating laboratories from 2006 to mid-2007 (Table 1). At the end of 2007, one laboratory reported the use of an in-house-developed real-time PCR assay, and by the end of 2012, 21 laboratories reported using several different in-house assays. In the middle of 2008, one laboratory replaced the Roche Amplicor HIV-1 DNA Test v1.5 with a Roche Cobas AmpliPrep/Cobas TaqMan platform (Roche, Indianapolis IN) (Table 1), and by the end of 2012, 25 laboratories were using the Cobas AmpliPrep/Cobas TaqMan HIV-1 Qual test. Furthermore, at the latest test cycle, two laboratories began using the Abbott m2000 (Abbott Laboratories, Abbott Park, IL), a high-throughput, automated extraction platform, and the Abbott RealTime HIV-1 Qualitative assay (Abbott Laboratories, Abbott Park, IL). Encouragingly, despite the move to other testing platforms, including the moderate increase in in-house-developed assays, the mean PT results were greater than 96%.
TABLE 1.
EID testing assays
| Panel | Roche Amplicor HIV-1 DNA Test, v1.5 |
Roche CAP-CTMa |
In-house real-time PCR |
Abbott RealTimeb |
||||
|---|---|---|---|---|---|---|---|---|
| No. of laboratoriesc | Mean score (%) | No. of laboratories | Mean score (%) | No. of laboratories | Mean score (%) | No. of laboratories | Mean score (%) | |
| 2006-2 | 17 | 93 | ||||||
| 2006-3 | 23 | 96 | ||||||
| 2007-1 | 22 | 97 | ||||||
| 2007-2 | 33 | 95 | ||||||
| 2007-3 | 42 | 98 | 1 | 100 | ||||
| 2008-1 | 38 | 98 | 1 | 100 | ||||
| 2008-2 | 55 | 97 | 1 | 100 | 3 | 100 | ||
| 2008-3 | 64 | 99 | 1 | 100 | 2 | 100 | ||
| 2009-1 | 66 | 96 | 3 | 100 | 3 | 100 | ||
| 2009-2 | 72 | 99 | 4 | 97 | 5 | 96 | ||
| 2009-3 | 62 | 100 | 6 | 100 | 5 | 100 | ||
| 2010-1 | 68 | 99 | 13 | 99 | 6 | 100 | ||
| 2010-2 | 60 | 100 | 17 | 96 | 9 | 98 | ||
| 2010-3 | 66 | 99 | 21 | 97 | 9 | 100 | ||
| 2011-1 | 65 | 100 | 20 | 98 | 18 | 99 | ||
| 2011-2 | 68 | 99 | 22 | 99 | 21 | 100 | ||
| 2011-3 | 62 | 97 | 23 | 97 | 16 | 100 | ||
| 2012-1 | 74 | 99 | 26 | 98 | 17 | 99 | ||
| 2012-2 | 71 | 100 | 29 | 98 | 21 | 100 | ||
| 2012-3 | 61 | 99 | 25 | 96 | 21 | 100 | 2 | 100 |
Roche CAP-CTM, Roche Cobas Ampliprep/Cobas TaqMan HIV-1 Qual.
Abbott RealTime, Abbott RealTime HIV-1 qualitative assay.
No. of laboratories, number of laboratories performing the assay and reporting results.
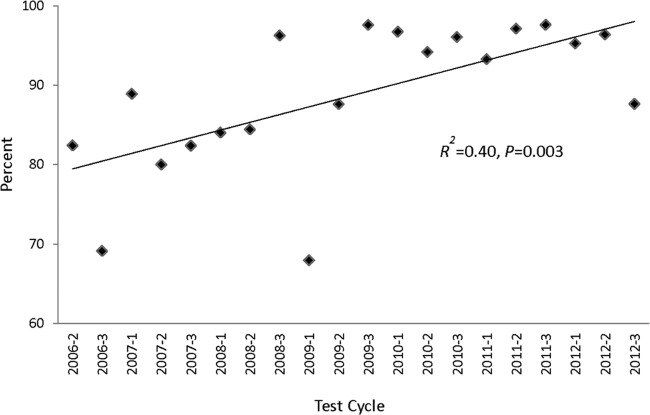
The performance of individual laboratories in PT is expected to improve as they analyze substandard results and institute corrective actions. The laboratories were categorized by the number of test cycles in which they participated in the program, and mean scores were determined. Regardless of the number of cycles in which laboratories participated, the mean scores were similar and no trend was observed (R2 = 0.06; P = 0.74) (data not shown). However, the percentage of laboratories scoring 100% at each of the 20 test cycles improved over time (R2 = 0.4; P = 0.003) (Fig. 2). Those laboratories that participated in all 20 test cycles scored an average of 99%, which is slightly better than the overall mean score of 98.4%.
FIG 2.
Percentage of laboratories scoring 100% at each test cycle. A linear regression for trend was used to analyze the percentages of laboratories scoring 100% over time.
Over the nearly 7-year period of evaluation (20 test cycles), more than 1,550 panels were distributed, among which 1,432 were completed, and 50.7% (69/136) of the laboratories scored 100% on all testing cycles in which they participated, corresponding to a median of 8 cycles (interquartile range [IQR], 3.5 to 11.5) (data not shown). For the 69 laboratories that scored 100% on all panels, they reported results on 553 panels. The other 67 laboratories accounted for all of the testing errors and completed a total of 879 panels; 743 (84.5%) were scored 100% and the remaining 136 panels received correct percentages ranging from 0% to 90%. Only 51 of 1,432 (3.6%) panels were scored 80% or less, and 31 laboratories received these scores. Of those 31 laboratories, only 12 scored 80% or less on more than one occasion. Overall, the mean score for the participating laboratories was 98.4% and the median number of cycles was 11 (IQR, 5.25 to 15).
The percentage of reported incorrect results is shown in Fig. 3. False-negative and false-positive findings as well as equivocal and indeterminate results were reported. Equivocal results were reported only for the Amplicor HIV-1 test when the final plate reader optical density value was 0.2 to 0.8. Indeterminate results were reported for the Cobas AmpliPrep/Cobas TaqMan HIV-1 Qual test. All of the blind specimens were expected to be either positive or negative for HIV-1 DNA. Except for the first testing cycle (2006-2) where all of the errors were found to be false negatives with the positive samples with the lowest HIV copy numbers, the range of reported incorrect results was typically 0% to 2.5% for all categories. Discordant values were recognized to represent transcriptional errors, post-PCR contamination, or improper interpretation of results. The testing laboratories were just as likely to report false-negative as false-positive results (1.28% versus 0.99%). Interestingly, despite accounting for only 14.7% of all the reported results, Cobas AmpliPrep/Cobas TaqMan HIV-1 Qual test users were 2.5 times more likely to report false-positive than false-negative results and accounted for nearly one-third (33 of 106) of all the false-positive results.
FIG 3.
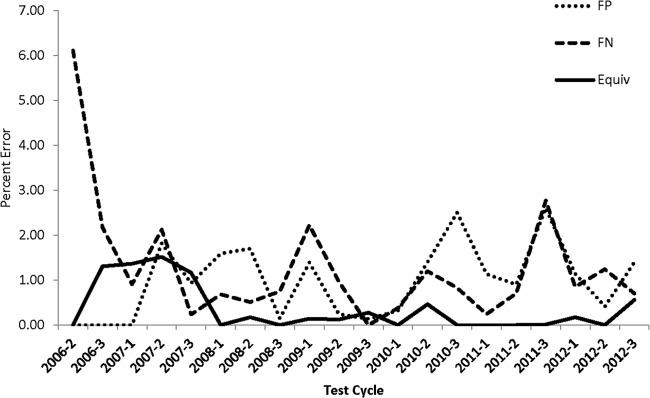
Percentage of discordant results by all participating laboratories. Discordant results included false-positive (FP), false-negative (FN), and combined equivocal and invalid (Equiv) results. Equivocal results (optical density value between 0.2 and 0.8) were reported only by Roche Amplicor DNA v1.5 users. Invalid results were reported by Cobas AmpliPrep/Cobas TaqMan HIV-1 Qual test users when the Amplilink software reported an invalid result(s) within a run where control values were valid.
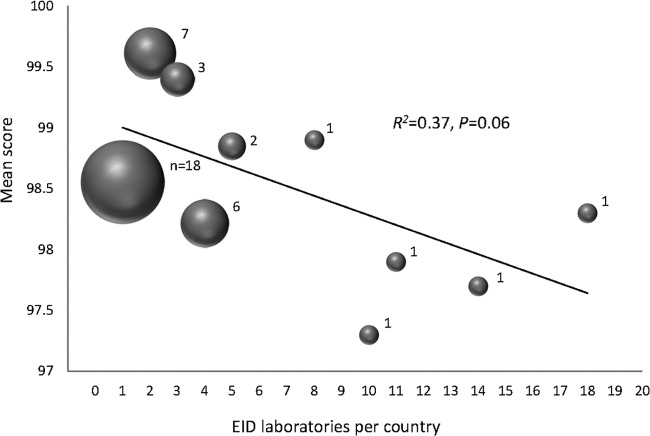
At the latest time point (2012-3), 61% (25 of 41) of the countries had only either 1 (18 countries) or 2 (7 countries) testing laboratories. Of the remaining 16 countries, 3 countries have 3, 6 countries have 4, 2 countries have 5, and 5 countries have 8, 10, 11, 14, and 18 testing laboratories. It was observed that as countries scaled up and added testing facilities to decentralize DNA PCR services to accommodate primarily logistical needs such as increased number of EID tests and a regional approach, there appeared to be an overall trend for lower-quality results (R2 = 0.37; P = 0.059) (Fig. 4). The countries with 10 or more EID PT participating laboratories scored on average 97.8 (range, 97.3 to 98.3).
FIG 4.
Mean scores according to number of laboratories in country at the 2012-3 test cycle. The 41 countries have 1, 2, 3, 4, 5, 8, 10, 11, 14, or 18 laboratories performing DNA PCR for EID. The bubble size corresponds to the number of countries. A linear regression for trend was used to analyze the mean scores per number of laboratories per country.
DISCUSSION
As EID testing began rapidly expanding worldwide in the past decade, there was an urgent need for an EQA program to monitor the quality of HIV DNA PCR testing using DBSs. A simple approach was developed to help participating laboratories monitor the quality of PCR testing for EID in low- and middle-income countries by the CDC headquarters. There is no cost to the participating laboratory, and the program consists of the provision of (i) a PT panel, (ii) known HIV-positive and -negative DBS specimens to be used as internal controls, and (iii) PT analysis and report results for end users.
Advances by low- and middle-income countries to conduct EID in infants have resulted in the expansion of the number of HIV-exposed children being identified and referred to treatment. To meet the increasing volume of EID testing, several sub-Saharan African countries have transitioned to using an automated extraction platform such as Roche Cobas AmpliPrep and real-time determination with Cobas TaqMan. With the switch to automation, there are expectations that analytical stage errors will be reduced because in recent decades, standardization, automation, and technological advances have significantly improved the analytical reliability of laboratory results (35). However, there is always room for improvement, particularly in pre- and postanalytical areas, and for more effective procedures for quality assessment and control. The routine use of quality control specimens as part of a quality assurance program prompted CDC to provide known HIV-positive and -negative DBS (DBS internal controls) with each PT panel.
Implementing a comprehensive laboratory quality management system and following good laboratory practices can reduce the frequency of testing errors associated with molecular diagnostic testing such as multistep DNA or RNA PCR. The variability of test results and the frequency of errors can be reduced with a dedicated comprehensive quality management system. This typically includes, at a minimum, site supervision, retesting of specimens (if possible), and participation in routine PT, all key components of laboratory accreditation. PT is a widely used approach to monitor the quality of testing and can result in increased accuracy of results and increased confidence by health care providers (36). For example, participation in an external CD4 proficiency test program (Canadian QASI-Quality Management System) resulted in a reduction in errors by 26% to 38% in simply 3 rounds of testing in RLS (37).
Many HIV-infected infants and children are either never diagnosed properly or lost to follow-up before enrollment into appropriate care and treatment programs. A poor-quality DNA PCR test or a long TAT could be potentially useless as an HIV-infected infant would not get referred to care and treatment and subsequent successful initiation of ART. Therefore, our PT program requires that laboratories treat the PT DBSs as if they were clinical samples and return the results electronically (fax or email) to CDC Atlanta within 30 days to be consistent with the 30-day TAT recommended by WHO (38). However, we observed routinely in all test cycles that nearly 10% or more of the laboratories failed to return the results within 30 days. The failure to meet the 30-day TAT requirement indicates a critical deficiency of laboratory practices because the laboratory testing is only a single component in an EID program. The entire EID process of proper specimen collection, DBS transport to the laboratory from the clinic, DNA PCR, timely return of the result to site, and return of the result to the caregiver should be completed within 30 days to offer the best chance for the HIV-positive infant to be linked to life-saving care and treatment. There were several obstacles to returning reliable PT results in a timely manner for many of the participating laboratories. These included specimen transportation delays, inconsistent power sources, reagent procurement delays and stock outages, expired test kits, and inadequate training and low wages for laboratory workers. All of these issues were reported as reasons for not providing PT test results in a timely manner, and these factors may lead to producing more unacceptable results (39).
Laboratories that scored less than 80% were contacted for remediation and corrective actions. Discordant values were attributed to transcriptional errors, post-PCR contamination, performance of the wrong test, and/or improper interpretation of results, which are consistent with previous reports (40) where frequent causes for failure in PT were clerical errors. Encouragingly, most laboratories did not treat the PT DBSs as unique specimens and rotated the PT responsibility among the trained staff. These observed bad practices defeat the quality assurance process of detecting defects within the testing system and are discouraged by the CDC.
Incorporating test automation was expected to reduce the levels of errors but was not expected to eliminate them completely. It was also expected that prior to laboratories switching from one technology to the next, the laboratory staff would perform method validation and correlation studies to qualify the assay differences. Nearly 20% of the participating laboratories have transitioned from manual to automated extraction of nucleic acids using Roche Cobas AmpliPrep for EID to significantly reduce the number of manipulations by laboratory staff and random human errors during the analytic phase, but inaccuracies continued to be observed at each testing cycle. Based on the limited data, it is currently unclear at what stage of the testing process the several erroneous results were generated by users of the latest state-of-the-art automated technology. Testing errors could conceivably be introduced by cross-contamination during the manual cutting with scissors of DBS spots from the cards or the introduction of PCR contaminants found in or on the automated extraction platform. The automated platforms are also used routinely for HIV load testing, and if good laboratory practices are not followed by decontaminating the surfaces, there is potential for cross-contamination. Despite years of practicing quality management in U.S. laboratories and continuous improvement in technology, errors for all clinical laboratory analytic testing (not just PCR) are high, estimated to range between 7% and 12% (40, 41). The need for good laboratory practice in resource-rich settings and RLS is imperative, despite the advances in technology. A major shortcoming throughout the world may be inadequate resources dedicated to quality assurance and quality control activities for all laboratory testing and, perhaps even more so, for very sensitive molecular diagnostic tests involving PCR.
The upper limit on the total discordant results was generally less than 2.5% for any testing cycle with the exception of the very first PT panel; there was a higher rate of false-negative results at the 2006-2 cycle than at the subsequent time points. The elevated level of false-negative results was likely attributable to the PT specimens containing a relatively low DNA copy number (1,250 copies/ml or ∼25 DNA copies/6-mm-diameter disc). The 2006-2 testing cycle observation was primarily confined to laboratories using the Roche MagNA Pure LC System for nucleic acid extraction in conjunction with the Roche Amplicor assay. Discrepancies between manual nucleic acid extraction and MagNA Pure LC have been reported (42, 43). The laboratories using the MagNA Pure extraction of DNA evaluated whole-DBS specimens (13-mm-diameter discs or ∼125 DNA copies) from that time point forward, and there was no indication of false negatives from those laboratories after the adjustment to specimen volume was made.
With the increased demand for PT in EID, the next logical step is to transfer the operation of the DBS-based PT system to RLS. Through this technology transfer, many of the logistical difficulties in panel distribution and transportation delays due to problems with customs authorities would be overcome. With an in-country or regional panel production approach, countries must develop strong EQA programs to support the increase in testing demand and testing facilities. As is shown in Fig. 4, which displays a trend of decreasing PT scores with the increasing number of laboratories per country, this trend suggests that laboratories performing EID should participate in a robust in-country laboratory network with EQA to monitor their performance and evaluate themselves among other peers. It also demonstrates an additional need for countrywide monitoring of EID programs during the current scale-up EID testing to reduce the potential for lower-quality results. Furthermore, PT programs are quite effective as an EQA tool in identifying poorly performing test sites (23).
Very few clinical laboratories in sub-Saharan Africa have received international accreditation (44), and this includes most of the laboratories participating in our PT program. The large increase in laboratory participation and the number of countries involved demonstrate the need for a cost-free PT program for laboratories performing HIV EID in RLS. Many of the PEPFAR-supported laboratories, which are struggling with inadequate resources, are encouraged to continually improve overall management and operations and seek accreditation from the new WHO Regional Office for Africa Program for Quality Improvement (45). This WHO Regional Office for Africa approach uses a novel five-step process to allow laboratories to gradually move toward accreditation in a cost-effective manner that positively reinforces progress at each step. One of the requirements for accreditation is participation in a PT program for each assay used in patient diagnostics and monitoring. Laboratory accreditation will help strengthen the laboratory and health system in these disease-burdened countries.
The need to test more infants and young children for HIV infection in low- and middle-income countries has led to advances in EID testing, and more laboratories are participating in EQA. The CDC PT program has expanded significantly to include 136 laboratories performing EID testing in 41 countries. Despite pressure to test more HIV-exposed children and significantly expand EID programs, there has been a significantly greater percentage of laboratories scoring 100% at each PT cycles and mean PT scores have improved over time. Discordant test results still occur but at a rate of no higher than 2.5%. Encouragingly, the positive effect of proficiency testing on the testing performance of the participating laboratories and the standardization of good laboratory practices should translate into laboratories improving their testing accuracy and have a definitive impact on EID testing quality.
ACKNOWLEDGMENTS
We thank the PT participants and the CDC International Laboratory Molecular Monitoring team.
We declare no conflicts of interest.
Use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention. The findings and conclusions in this report are ours and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 18 December 2013
REFERENCES
- 1.UNAIDS 2010. UNAIDS report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland [Google Scholar]
- 2.Berk DR, Falkovitz-Halpern MS, Hill DW, Albin C, Arrieta A, Bork JM, Cohan D, Nilson B, Petru A, Ruiz J, Weintrub PS, Wenman W, Maldonado YA. 2005. Temporal trends in early clinical manifestations of perinatal HIV infection in a population-based cohort. JAMA 293:2221–2231. 10.1001/jama.293.18.2221 [DOI] [PubMed] [Google Scholar]
- 3.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA. 2008. Early antiretroviral therapy and mortality among HIV-infected infants. N. Engl. J. Med. 359:2233–2244. 10.1056/NEJMoa0800971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO 2010. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 5.Sherman GG, Driver GA, Coovadia AH. 2008. Evaluation of seven rapid HIV tests to detect HIV-exposure and seroreversion during infancy. J. Clin. Virol. 43:313–316. 10.1016/j.jcv.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 6.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. 2005. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J. Acquir. Immune Defic. Syndr. 38:615–617. 10.1097/01.qai.0000143604.71857.5d [DOI] [PubMed] [Google Scholar]
- 7.Nyambi PN, Fransen K, De Beenhouwer H, Chomba EN, Temmerman M, Ndinya-Achola JO, Piot P, van der Groen G. 1994. Detection of human immunodeficiency virus type 1 (HIV-1) in heel prick blood on filter paper from children born to HIV-1-seropositive mothers. J. Clin. Microbiol. 32:2858–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassol S, Salas T, Gill MJ, Montpetit M, Rudnik J, Sy CT, O'Shaughnessy MV. 1992. Stability of dried blood spot specimens for detection of human immunodeficiency virus DNA by polymerase chain reaction. J. Clin. Microbiol. 30:3039–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassol SA, Lapointe N, Salas T, Hankins C, Arella M, Fauvel M, Delage G, Boucher M, Samson J, Charest J, et al. 1992. Diagnosis of vertical HIV-1 transmission using the polymerase chain reaction and dried blood spot specimens. J. Acquir. Immune Defic. Syndr. 5:113–119 [PubMed] [Google Scholar]
- 10.Biggar RJ, Miley W, Miotti P, Taha TE, Butcher A, Spadoro J, Waters D. 1997. Blood collection on filter paper: a practical approach to sample collection for studies of perinatal HIV transmission. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 14:368–373. 10.1097/00042560-199704010-00010 [DOI] [PubMed] [Google Scholar]
- 11.Comeau AM, Pitt J, Hillyer GV, Landesman S, Bremer J, Chang BH, Lew J, Moye J, Grady GF, McIntosh K. 1996. Early detection of human immunodeficiency virus on dried blood spot specimens: sensitivity across serial specimens. Women and Infants Transmission Study Group. J. Pediatr. 129:111–118 [DOI] [PubMed] [Google Scholar]
- 12.Dunn DT, Brandt CD, Krivine A, Cassol SA, Roques P, Borkowsky W, De Rossi A, Denamur E, Ehrnst A, Loveday C. 1995. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contributions of intra-uterine and intra-partum transmission. AIDS 9:F7–F11 [DOI] [PubMed] [Google Scholar]
- 13.Rollins NC, Dedicoat M, Danaviah S, Page T, Bishop K, Kleinschmidt I, Coovadia HM, Cassol SA. 2002. Prevalence, incidence, and mother-to-child transmission of HIV-1 in rural South Africa. Lancet 360:389. 10.1016/S0140-6736(02)09599-5 [DOI] [PubMed] [Google Scholar]
- 14.Sherman GG, Jones SA, Coovadia AH, Urban MF, Bolton KD. 2004. PMTCT from research to reality—results from a routine service. S. Afr. Med. J. 94:289–292 [PubMed] [Google Scholar]
- 15.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, Bolton KD. 2005. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr. Infect. Dis. J. 24:993–997. 10.1097/01.inf.0000187036.73539.8d [DOI] [PubMed] [Google Scholar]
- 16.Lilian RR, Kalk E, Bhowan K, Berrie L, Carmona S, Technau K, Sherman GG. 2012. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J. Clin. Microbiol. 50:2373–2377. 10.1128/JCM.00431-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilian RR, Kalk E, Technau KG, Sherman GG. 2013. Birth diagnosis of HIV infection in infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr. Infect. Dis. J. 32:1080–1085. 10.1097/INF.0b013e318290622e [DOI] [PubMed] [Google Scholar]
- 18.Chalermchan W, Pitak S, Sungkawasee S. 2007. Evaluation of Thailand national external quality assessment on HIV testing. Int. J. Health Care Qual. Assur. 20:130–140. 10.1108/09526860710731825 [DOI] [PubMed] [Google Scholar]
- 19.Goguel AF. 1991. HBV and HIV serological markers: the National External Quality Assessment Scheme in France. Ann. Ist. Super. Sanita 27:511–515 [PubMed] [Google Scholar]
- 20.Hannon WH, Lewis DS, Jones WK, Powell MK. 1989. A quality assurance program for human immunodeficiency virus seropositivity screening of dried-blood spot specimens. Infect. Control Hosp. Epidemiol. 10:8–13. 10.1086/645908 [DOI] [PubMed] [Google Scholar]
- 21.Hofherr LK, Peddecord KM, Benenson AS, Garfein RS, Francis DP, Ferran KL, Taylor RN. 1992. Methods for a model blind proficiency testing system. Clin. Lab. Sci. 5:160–164 [PubMed] [Google Scholar]
- 22.Jackson JB, Drew J, Lin HJ, Otto P, Bremer JW, Hollinger FB, Wolinsky SM. 1993. Establishment of a quality assurance program for human immunodeficiency virus type 1 DNA polymerase chain reaction assays by the AIDS Clinical Trials Group. ACTG PCR Working Group, and the ACTG PCR Virology Laboratories. J. Clin. Microbiol. 31:3123–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peddecord KM, Benenson AS, Hofherr LK, Francis DP, Garfein RS, Ferran KL, Taylor RN, Schalla WO, Ascher MS. 1992. Analytic results of HIV-1 testing using blind proficiency testing. Clin. Lab. Sci. 5:165–171 [PubMed] [Google Scholar]
- 24.Reichelderfer PS, Jackson JB. 1994. Quality assurance and use of PCR in clinical trials. PCR Methods Appl. 4:S141–S149. 10.1101/gr.4.3.S141 [DOI] [PubMed] [Google Scholar]
- 25.Rickman WJ, Monical C, Waxdal MJ. 1993. Improved precision in the enumeration of absolute numbers of lymphocyte phenotypes with long-term monthly proficiency testing. Ann. N. Y. Acad. Sci. 677:53–58. 10.1111/j.1749-6632.1993.tb38764.x [DOI] [PubMed] [Google Scholar]
- 26.Schalla WO, Hearn TL, Taylor RN, Eavenson E, Valdiserri RO, Essien JD. 1990. CDC's Model Performance Evaluation Program: assessment of the quality of laboratory performance for HIV-1 antibody testing. Public Health Rep. 105:167–171 [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz JS, Dans PE, Kinosian BP. 1988. Human immunodeficiency virus test evaluation, performance, and use. Proposals to make good tests better. JAMA 259:2574–2579 [PubMed] [Google Scholar]
- 28.Yen-Lieberman B, Brambilla D, Jackson B, Bremer J, Coombs R, Cronin M, Herman S, Katzenstein D, Leung S, Lin HJ, Palumbo P, Rasheed S, Todd J, Vahey M, Reichelderfer P. 1996. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J. Clin. Microbiol. 34:2695–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweiger B, Pauli G, Zeichhardt H, Kucherer C. 1997. A multicentre quality assessment study to monitor the performance of HIV-1 PCR. J. Virol. Methods 67:45–55. 10.1016/S0166-0934(97)00075-X [DOI] [PubMed] [Google Scholar]
- 30.Folks T, Benn S, Rabson A, Theodore T, Hoggan MD, Martin M, Lightfoote M, Sell K. 1985. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc. Natl. Acad. Sci. U. S. A. 82:4539–4543. 10.1073/pnas.82.13.4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC 1995. Guidelines for the shipment of dried blood spot specimens. CDC, Atlanta, GA [Google Scholar]
- 32.IATA 2011. Division 6.2—infectious substances (DGR362). International Air Transport Association (IATA), Montreal, Canada [Google Scholar]
- 33.WHO 2010. Guidance on regulations for the transport of infectious substances 2011–2012 WHO, Geneva, Switzerland [Google Scholar]
- 34.Stevens W, Sherman G, Downing R, Parsons LM, Ou CY, Crowley S, Gershy-Damet GM, Fransen K, Bulterys M, Lu L, Homsy J, Finkbeiner T, Nkengasong JN. 2008. Role of the laboratory in ensuring global access to ARV treatment for HIV-infected children: consensus statement on the performance of laboratory assays for early infant diagnosis. Open AIDS J. 2:17–25. 10.2174/1874613600802010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stankovic AK. 2004. The laboratory is a key partner in assuring patient safety. Clin. Lab. Med. 24:1023–1035. 10.1016/j.cll.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 36.Glencross DK, Aggett HM, Stevens WS, Mandy F. 2008. African regional external quality assessment for CD4 T-cell enumeration: development, outcomes, and performance of laboratories. Cytometry B Clin. Cytom. 74(Suppl 1):S69–S79. 10.1002/cyto.b.20397 [DOI] [PubMed] [Google Scholar]
- 37.Bergeron M, Ding T, Houle G, Ares L, Chabot C, Soucy N, Seely P, Sherring A, Bogdanovic D, Faucher S, Summers R, Somorjai R, Sandstrom P. 2010. QASI, an international quality management system for CD4 T-cell enumeration focused to make a global difference. Cytometry B Clin. Cytom. 78:41–48. 10.1002/cyto.b.20487 [DOI] [PubMed] [Google Scholar]
- 38.WHO 2010. WHO recommendations on the diagnosis of HIV infection in infants and children. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 39.Delost MD, Miller WG, Chang GA, Korzun WJ, Nadder TS. 2009. Influence of credentials of clinical laboratory professionals on proficiency testing performance. Am. J. Clin. Pathol. 132:550–554. 10.1309/AJCPWCBSYISV1ASI [DOI] [PubMed] [Google Scholar]
- 40.Plebani M. 2006. Errors in clinical laboratories or errors in laboratory medicine? Clin. Chem. Lab. Med. 44:750–759. 10.1515/CCLM.2006.123 [DOI] [PubMed] [Google Scholar]
- 41.Kalra J. 2004. Medical errors: impact on clinical laboratories and other critical areas. Clin. Biochem. 37:1052–1062. 10.1016/j.clinbiochem.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 42.Schuurman T, van Breda A, de Boer R, Kooistra-Smid M, Beld M, Savelkoul P, Boom R. 2005. Reduced PCR sensitivity due to impaired DNA recovery with the MagNA Pure LC total nucleic acid isolation kit. J. Clin. Microbiol. 43:4616–4622. 10.1128/JCM.43.9.4616-4622.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton JC, Akkers E, Coovadia AH, Meyers TM, Stevens WS, Sherman GG. 2007. Evaluation of dried whole blood spots obtained by heel or finger stick as an alternative to venous blood for diagnosis of human immunodeficiency virus type 1 infection in vertically exposed infants in the routine diagnostic laboratory. Clin. Vaccine Immunol. 14:201–203. 10.1128/CVI.00223-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peter TF, Rotz PD, Blair DH, Khine AA, Freeman RR, Murtagh MM. 2010. Impact of laboratory accreditation on patient care and the health system. Am. J. Clin. Pathol. 134:550–555. 10.1309/AJCPH1SKQ1HNWGHF [DOI] [PubMed] [Google Scholar]
- 45.Gershy-Damet GM, Rotz P, Cross D, Belabbes el H, Cham HF, Ndihokubwayo JB, Fine G, Zeh C, Njukeng PA, Mboup S, Sesse DE, Messele T, Birx DL, Nkengasong JN. 2010. The World Health Organization African region laboratory accreditation process: improving the quality of laboratory systems in the African region. Am. J. Clin. Pathol. 134:393–400. 10.1309/AJCPTUUC2V1WJQBM [DOI] [PubMed] [Google Scholar]