FIG 1.
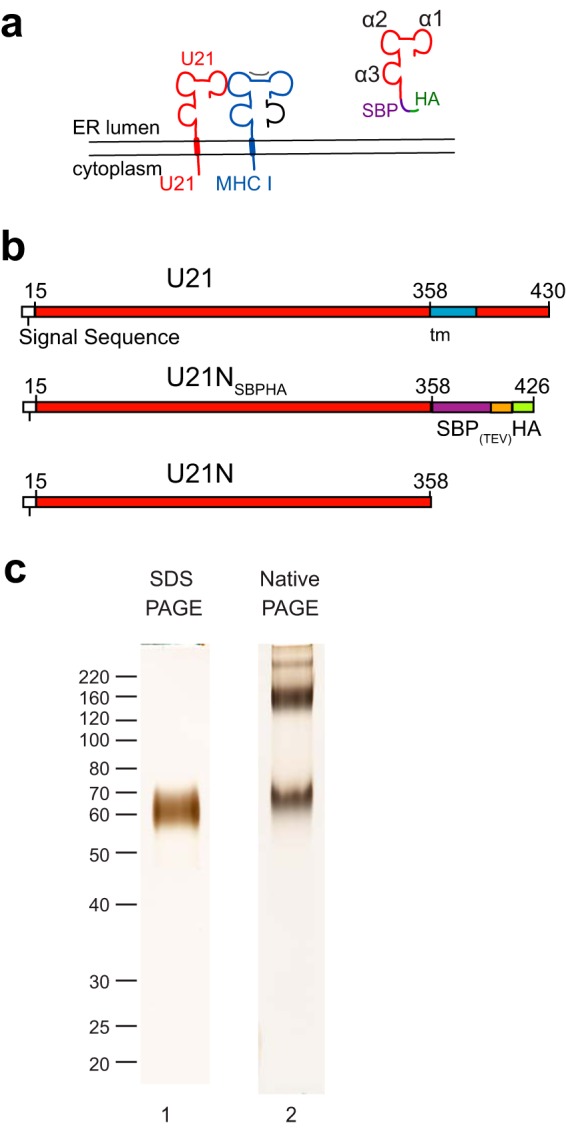
Purified U21 migrates as multiple species in native gels. (a) Topological schematic of the predicted similarity in structure between U21(red) and class I MHC, in which a class I MHC heavy chain (blue) is depicted bound to peptide (gray) and β2-microglobulin (black). Putative α1, α2, and α3 domains are noted in the cartoon depiction of U21NSBPHA. (b) Schematic of U21 constructs used for purification: top, full-length U21, with amino acid numbers, signal sequence (white), and transmembrane domain (blue); middle, soluble U21NSBPHA, with SBP tag (purple), TEV protease cleavage site (orange), and HA tag (green); bottom, U21N (or U21 lumenal domain) alone. (c) Lane 1, purified U21NSBPHA subjected to SDS-polyacrylamide gel electrophoresis and silver stained. Molecular weight size markers are noted. Lane 2, purified U21NSBPHA subjected to native gel electrophoresis and silver stained.
