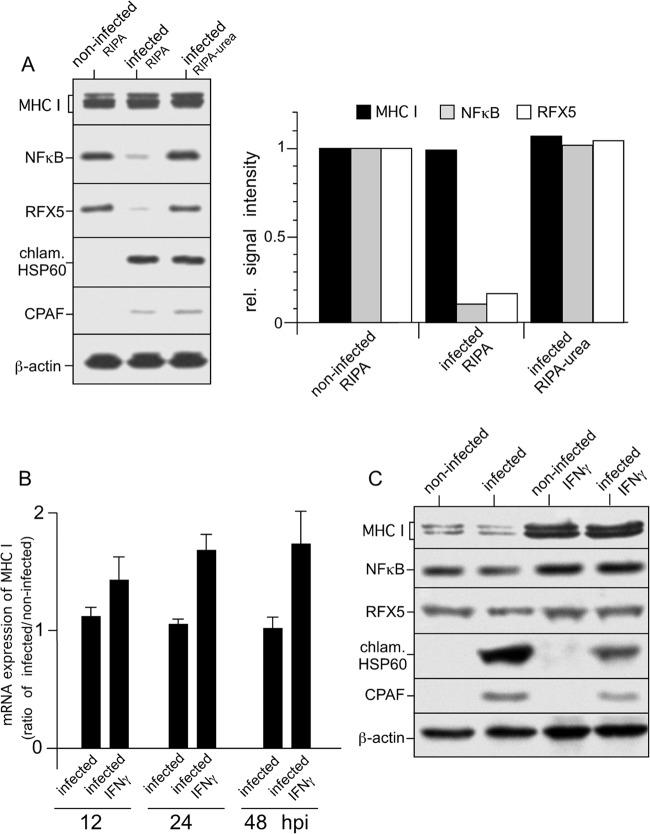
FIG 1.
Expression of MHC I in C. trachomatis serovar D-infected HeLa cells. (A) Proteolysis of NF-κB and RFX5 is dependent on cell processing and does not affect steady-state levels of MHC I heavy chains. Cells were infected at an MOI of 5, and samples were prepared at 48 hpi. Lysates of infected HeLa cells were prepared in RIPA buffer or by direct lysis in RIPA buffer containing 4 M urea, separated by SDS-PAGE, and probed with antibodies to MHC I, NF-κB, RFX5, chlamydial Hsp60 (chlam. HSP60), CPAF, and β-actin (loading control). Fluorographs were analyzed by densitometric scanning. rel., relative. (B) Levels of MHC I mRNA in infected cells. Samples were analyzed by real-time RT-PCR at 12, 24, and 48 hpi. Relative gene expression represents the ratio of mRNA levels in Chlamydia-infected and noninfected cells. Data were calculated as described in Materials and Methods. Values represent the mean of three independent experiments (with standard deviations). (C) Neither constitutive nor IFN-γ-inducible MHC I expression is affected in Chlamydia-infected cells. HeLa cells infected or not infected with Chlamydia were cultured with or without IFN-γ. Therefore, HeLa cells were pretreated with 100 IU of IFN-γ/ml for 48 h and also during the following 48 h of chlamydial infection. Lysates were prepared in RIPA buffer containing 4 M urea. MHC I, NF-κB, RFX5, chlamydial Hsp60, and CPAF from lysates of infected and noninfected HeLa cells were detected by Western blot assays with specific antibodies. β-Actin staining was used as a loading control and for standardization.