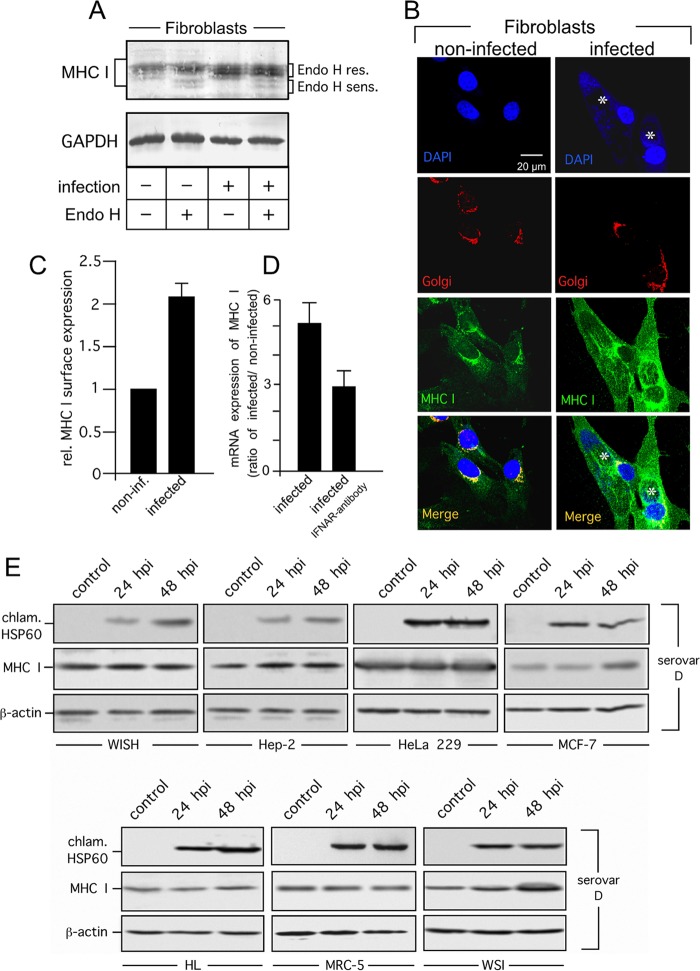
FIG 6.
MHC I expression and surface presentation in C. trachomatis serovar D-infected primary fibroblasts and human cell lines. (A) MHC I expression in infected fibroblasts. Cell lysates were treated with or without Endo H to distinguish between Endo H-resistant (res.) post-ER and Endo H-sensitive (sens.) ER forms of MHC I. Western blot assays were probed with antibodies to MHC I and GAPDH (loading control). (B) Colocalization of MHC I with the Golgi compartment in C. trachomatis serovar D-infected primary fibroblasts. Cell monolayers were costained with 4′,6-diamidino-2-phenylindole for DNA (nuclei and chlamydial inclusions, indicated by asterisks, blue), GM130 antibody (Golgi compartment, red), and MHC I antibody (green). (C) MHC I surface expression of fibroblasts determined by flow cytometry. Samples were prepared at 48 h after infection. Surface MHC I was stained with a fluorophore-conjugated antibody. C. trachomatis serovar D-positive cells in infected cultures were identified following intracellular staining with a FITC-conjugated antibody against C. trachomatis (see Fig. 5). Mean fluorescence intensities are depicted as histograms. Values represent the mean of three independent experiments (with standard deviations). (D) Expression of MHC I mRNA in infected and noninfected fibroblasts. Samples were analyzed by real-time RT-PCR. Relative (rel.) gene expression represents the ratio of the mRNA level in Chlamydia-infected cells to that in noninfected cells. Data were calculated as described in Materials and Methods. To neutralize IFN-β activity in fibroblast cultures, an antibody against type I IFN receptor (IFNAR) was added immediately after infection. (E) MHC I expression in C. trachomatis serovar D-infected WISH, Hep-2, HeLa 229, MCF-7, HL, MRC-5, and WSI cells. Lysates of infected (24 and 48 hpi) and noninfected (control) cells were separated by SDS-PAGE and analyzed by Western blotting with antibodies against MHC I, chlamydial Hsp60 (chlam. HSP60), and β-actin.