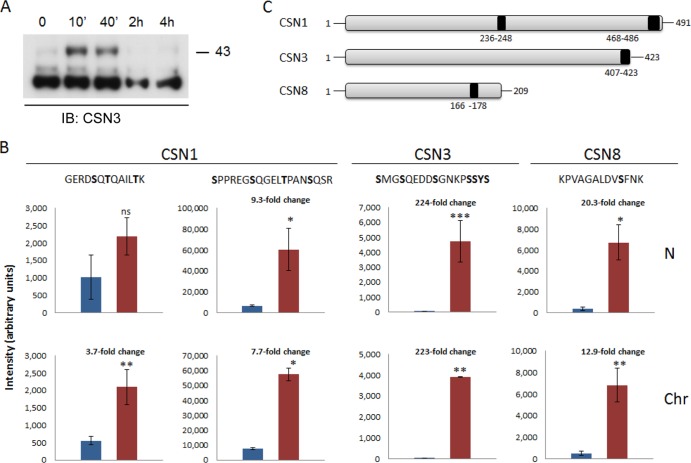
FIG 6.
UV damage induces upregulation of specific phophopeptides within the nucleus. (A) Phosphorylated CSN3 accumulates in the chromatin-bound fraction, following DNA damage. Cells were exposed to 20 J/m2 of UV-C light. After induction of DNA damage, cellular proteins were fractionated at different time points in the absence of phosphatase inhibitors and visualized using an anti-CSN3 antibody. In response to UV irradiation, more slowly migrating bands, which may correspond to a phosphorylated form of CSN3, transiently appeared in the chromatin-bound fraction. (B) Cells were exposed to 20 J/m2 of UV-C light. Cellular proteins were fractionated 10 min after induction of DNA damage and subjected to phosphopeptide analysis. The fold change of the phosphopeptide intensities of control (blue bars) versus UV-treated (red bars) phosphopeptides, in the nucleoplasmic (N) and chromatin-bound (Chr) fractions, is indicated. Significances were calculated using Student's t test; single asterisks, double asterisks, and triple asterisks designate significance at confidence levels of 0.01, 0.005, and 0.0001, respectively. ns, nonsignificant. Each value represents the average of the intensities with standard deviation of the same phosphopeptide in three biological replicates with three technical replicates each. The most probable phosphorylation site on each peptide, as calculated by the Mascot software, is highlighted in bold. (C) A schematic representation highlighting the positions of the upregulated phosphorylation sites within CSN1, CSN3, and CSN8.