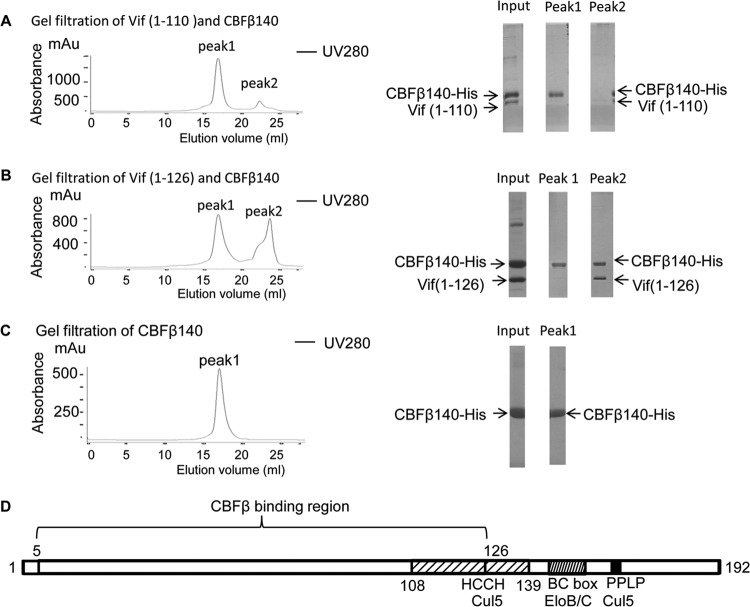
FIG 2.
The Vif C terminus up to residue 126 is required to form a stable complex with CBFβ. Vif residues 1 to 110 or Vif residues 1 to 126 and CBFβ residues 1 to 140 were purified by nickel pull-down and loaded onto Superdex 200 for gel filtration (left). The fractions were collected and subjected to SDS-PAGE, followed by Coomassie staining (right). (A) Gel filtration of Vif residues 1 to 110 and CBFβ. Vif did not elute with CBFβ. (B) Gel filtration of Vif residues 1 to 126 and CBFβ. Vif and CBFβ eluted in the same peak. (C) Gel filtration of CBFβ alone. mAu, milliabsorbance units. (D) Region important for the interaction between Vif and CBFβ.