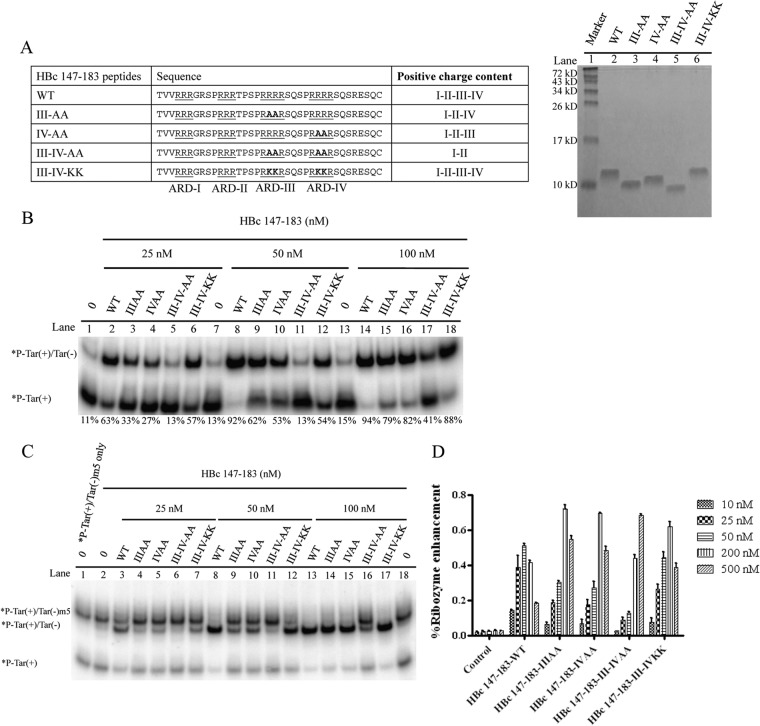
FIG 8.
Positive charge content of HBc ARD is important for nucleic acid chaperone activity. (A) Amino acid sequences of mutant HBc ARD peptides containing various arginine-to-alanine (R-to-A) or arginine-to-lysine (R-to-K) substitutions and characterization of HBc ARD peptides by Tricine SDS-PAGE. These peptides were stained with Green Angel. (B) The DNA annealing activity of HBc ARD peptides was significantly reduced by R-to-A substitutions (lanes 3 to 5 and 9 to 11) but not by R-to-K substitutions (lanes 6, 12, and 18). (C) The strand displacement activity of HBc ARD peptides was significantly reduced by R-to-A substitutions (lanes 4 to 6 and 9 to 11) but not by R-to-K substitutions (lanes 7, 12, and 17). (D) The enhancement of hammerhead ribozyme cleavage activity by HBc ARD peptides was significantly reduced by R-to-A substitutions, but not by R-to-K substitutions, at 25 and 50 nM.